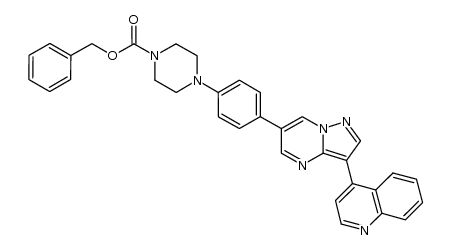
1062368-24-4
| 中文名 | 4-(6-(4-(哌嗪-1-基)苯基)吡唑并[1,5-a]嘧啶-3-基)喹啉 |
|---|---|
| 英文名 | 4-[6-(4-piperazin-1-ylphenyl)pyrazolo[1,5-a]pyrimidin-3-yl]quinoline |
| 中文别名 | 厚朴酚.木兰醇 |
| 英文别名 |
4-{6-[4-(1-Piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl}quinoline
4-[6-[4-(1-Piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline hydrochloride DM-3189 Quinoline, 4-[6-[4-(1-piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]- UNII-W69H5YQU9O cc-233 S2618_Selleck LDN193189 6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine |
| 描述 | LDN193189是 BMP 信号传导抑制剂,抑制ALK1,ALK2,ALK3和ALK6的IC50分别为0.8,0.8,5.3,16.7 nM。 |
|---|---|
| 相关类别 | |
| 靶点 |
IC50: 5 nM (ALK2), 30 nM (ALK3)[1] |
| 体外研究 | LDN-193189以比dorsomorphin更强的效力抑制BMP4介导的Smad1,Smad5和Smad8活化(IC50 = 5 nM对470 nM),同时保留200倍于BMP信号传导与TGF-β信号传导的选择性(TGF-β的IC50≥1,000)纳米)。 LDN-193189有效抑制BMP I型受体ALK2和ALK3的转录活性(IC50分别为5 nM和30 nM),对激活素和TGF-βⅠ型受体ALK4,ALK5和ALK7的作用显着较弱(IC50≥与母体化合物相比,相对于AMP活化的蛋白激酶,PDGFR和MAPK信号传导途径,BMP信号传导的选择性增加(500nM)。 LDN-193189阻断由组成型活性ALK2R206H或ALK2Q207D突变蛋白诱导的转录活性。这些发现表明LDN-193189可能影响BMP诱导的成骨细胞分化。事实上,即使在BMP刺激后12小时施用,LDN-193189也抑制BMP4对C2C12细胞中碱性磷酸酶活性的诱导,表明成骨分化需要持续的BMP信号传导活性[1]。 |
| 体内研究 | 在第一个实验中,在植入后7天肿瘤变得可触知后,每天两次腹膜内注射LDN-193189(3mg/kg)。对照载体和LDN-193189处理的小鼠之间的生长速率在前5周后没有显着差异,但是在处理后6和7周后检测到生长速率的差异。在第二个实验中,从PCa-118b肿瘤中分离细胞并皮下注射到SCID小鼠(每只小鼠1×10 6个细胞)中。在肿瘤可触知之前,在肿瘤细胞注射后5天将LDN-193189或载体应用于小鼠。通过肿瘤大小测量的这两组之间的平均生长速率的差异在治疗后6周和7周是显着的。在第7周研究结束时,肿瘤重量也显示出显着差异。肿瘤的X射线显示LDN-193189治疗组的肿瘤中异位骨体积和骨密度与对照组相比均降低[2]。来自肺动脉高压(PAH)大鼠的肺动脉平滑肌细胞(PASMCs)与西地那非和LDN-193189的共同孵育完全抑制了骨形态发生蛋白(BMPR2)和Cx40表达的抗增殖和上调。西地那非[3]。 |
| 激酶实验 | 将C2C12细胞以每孔2,000个细胞接种到96孔板中,在补充有2%FBS的DMEM中。用BMP配体和LDN-193189或载体将孔一式四份处理。在培养6天后,在50μLTris缓冲盐水和1%Triton X-100中收集细胞。将裂解物加入到96孔板中的对硝基苯基磷酸酯试剂中1小时,然后评估碱性磷酸酶活性(405nm处的吸光度)。使用与用于碱性磷酸酶测量的那些相同处理的重复孔,通过Cell Titer Aqueous One(490nm处的吸光度)测量细胞活力和数量。 |
| 细胞实验 | 将生长的小鼠PASMC在含有0.3μgId1启动子荧光素酶报告基因构建体(BRE-Luc)和0.6μg表达组成型活性形式的BMP I型受体(caALK2,caALK3或caALK6)的质粒的六孔板中瞬时转染至50%汇合。 ,使用Fugene6。为了评估活化素和TGF-βI型受体功能,用0.3μgPAI1(纤溶酶原激活物抑制剂-1)启动子荧光素酶报告基因构建体(CAGA-Luc)与0.6μg表达I型组成型活性形式的质粒瞬时转染PASMCs。受体(caALK4,caALK5和caALK7)。对于两种报告质粒,使用0.2μgpRL-TKRenilla荧光素酶来控制转染效率。在转染后1小时开始,将PASMC与LDN-193189(2nM-32μM)或载体一起温育。通过双荧光素酶测定试剂盒,通过萤火虫与海肾荧光素酶活性的比率收获细胞提取物并定量相对启动子活性。 |
| 动物实验 | 小鼠[2]在第一个实验中,SCID小鼠植入MDA-PCa-118b肿瘤。在肿瘤达到可测量的大小7天后,给小鼠注射LDN-193189(3mg / kg)或每天两次腹膜内注射载体。每周测量肿瘤大小和体重。在处死前三天和一天给小鼠注射钙黄绿素。收集血液并称重肿瘤。使用OsteoMeasure分析系统将一部分肿瘤固定在甲醛中用于微型计算机断层扫描(microCT),或使用OsteoMeasure分析系统进一步脱钙用于骨组织形态学分析,或快速冷冻用于RNA制备。通过ELISA测定小鼠血清中的骨钙蛋白。在第二个实验中,首先用Accumax消化PCa-118b肿瘤,将分离的细胞接种过夜,以1:1的比例重悬于基质胶中,并皮下注射到SCID小鼠(1×106细胞/小鼠)中。注射后5天用LDN-193189处理小鼠。大鼠[3]雄性Sprague-Dawley(SD)大鼠,8周龄,体重200-220g,购自南京医科大学动物中心。将大鼠随机分配到七个实验组中的一个(每组n = 6)。在自然12/12小时昼/夜循环下,大鼠可以自由获取食物和水。通过皮下注射到背部区域给予大鼠野百合碱(60mg / kg)。在血液动力学评估后,在研究的第28天收获动物的肺。西地那非组在给予MCT(60mg / kg)后每天接受西地那非的胃内给药。 LDN-193189组每天胃内给予西地那非(50mg / kg)和腹膜内注射LDN-193189(10mg / kg)。在其他组中,给予相同体积的盐水。 |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 分子式 | C25H22N6 |
| 分子量 | 406.482 |
| 精确质量 | 406.190582 |
| PSA | 58.35000 |
| LogP | 1.81 |
| 外观性状 | yellow to orange |
| 折射率 | 1.740 |
| 储存条件 | ?20°C |
| 水溶解性 | H2O: soluble5mg/mL, clear (warmed) |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: LDN193189 dihydrochloride REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. This substance is not classified as dangerous according to Directive 67/548/EEC. Label elements This substance is not classified as dangerous according to Directive 67/548/EEC. Other hazards - none SECTION 3: Composition/information on ingredients Substances Synonyms: 4-[6-[4-(1-Piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline dihydrochloride Formula: C25H22N6.2HCl Molecular Weight: 479,40 g/mol No components need to be disclosed according to the applicable regulations. SECTION 4: First aid measures Description of first aid measures no data available Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media no data available Special hazards arising from the substance or mixture Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas Advice for firefighters no data available Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures For personal protection see section 8. Environmental precautions no data available Methods and materials for containment and cleaning up no data available Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Recommended storage temperature: -20 °C Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls no data available SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability no data available Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: Not available To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods no data available SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15: Regulatory information This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006. Safety, health and environmental regulations/legislation specific for the substance or mixture no data available Chemical Safety Assessment For this product a chemical safety assessment was not carried out SECTION 16: Other information |
| 海关编码 | 29339900 |
|---|
|
~88% 
1062368-24-4 |
| 文献:THE GENERAL HOSPITAL CORPORATION; THE BRIGHAM AND WOMEN'S HOSPITAL, INC. Patent: WO2009/114180 A1, 2009 ; Location in patent: Page/Page column 68-69 ; |
| 上游产品 1 | |
|---|---|
| 下游产品 0 | |