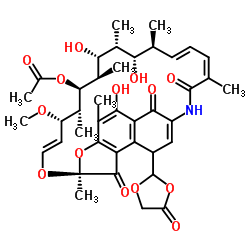
13553-79-2
| 中文名 | 利福霉素-S |
|---|---|
| 英文名 | Rifamycin S |
| 中文别名 |
利福霉素 S
利福霉素S 利福霉素 利福平霉素 |
| 英文别名 |
Rifamycin,1,4-dideoxy-1,4-dihydro-1,4-dioxo
UNII-PI53N820JV rifomycin-S rifamycin-S (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17-Trihydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6,23,27,29-tetraoxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),2,4,9,19,21,25-heptaen-13-yl acetate NCI 144-130 (2S,12Z,14E,16S,17S,18R,19R,20R,21S,22R,23S,24E)-5,17,19-trihydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-1,6,9,11-tetraoxo-1,2,6,9-tetrahydro-2,7-(epoxypentadeca[1,11,13]trienoimino)naphtho[2,1-b]furan-21-yl acetate EINECS 236-938-4 2,7-(Epoxy[1,11,13]pentadecatrienoimino)naphtho[2,1-b]furan-1,6,9,11(2H)-tetrone, 21-(acetyloxy)-5,17,19-trihydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-, (2S,12Z,14E,16S,17S,18R,19R,20R,21S,22R,23S,24E)- (12S,3E,5S,13E,15Z)-7t-acetoxy-15,9c,11t-trihydroxy-5r-methoxy-12,4,6t,8c,10c,12t,16-heptamethyl-2-oxa-18-aza-1(2,7)-naphtho[2,1-b]furana-cyclooctadecaphane-3,13,15-triene-11,6,9,17-tetraone rifaximin S O1,O4-didehydro-rifamycin (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17-Trihydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6,23,27,29-tetraoxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),2,4,9 ,19,21,25-heptaen-13-yl acetate 1,4-Dideoxy-1,4-dihydro-1,4-dioxorifamycin RifamycinS |
| 描述 | Rifamycin S 是一种醌和抗革兰氏阳性细菌 (包括 MRSA) 的抗生素。Rifamycin S 是涉及两个电子的可逆氧化还原系统的氧化形式。Rifamycin S 可以产生活性氧 (ROS) 并抑制微粒体脂质过氧化,并可用于肺结核和麻风病的研究。 |
|---|---|
| 相关类别 | |
| 靶点 |
Gram-positive bacteria[3] Reactive oxygen species (ROS)[1] |
| 体外研究 | 利福霉素SV对细菌生长的抑制作用是由于细胞内的氧化还原循环产生活性氧。金属离子Mn2+、Cu2+、Co2+诱导利福霉素SV氧化为利福霉素S。最有效的金属离子是Mn2+[2]。 |
| 体内研究 | 大鼠肝线粒体亚颗粒在NADH和利福霉素存在下也产生羟基自由基。NADH脱氢酶(复合物I)是参与利福霉素S还原的主要成分。与NADPH相比,NADH在催化这些抗生素与大鼠肝微粒体相互作用方面几乎同样有效(利福霉素S)。利福霉素S很容易被铁(II)还原成相应的对苯二酚利福霉素SV。利福霉素S形成可检测的Fe(II)-(利福霉素S)3复合物。Fe:ATP诱导的脂质过氧化被利福霉素S完全抑制。利福霉素S可与大鼠肝微粒体相互作用,进行氧化还原循环,当存在铁络合物时,随后产生羟基自由基[1]。 |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 917.4±65.0 °C at 760 mmHg |
| 熔点 | 179-181ºC (dec.) |
| 分子式 | C37H45NO12 |
| 分子量 | 695.753 |
| 闪点 | 508.6±34.3 °C |
| 精确质量 | 695.294189 |
| PSA | 194.99000 |
| LogP | 2.87 |
| 外观性状 | 黄色橙色结晶粉末 |
| 蒸汽压 | 0.0±0.3 mmHg at 25°C |
| 折射率 | 1.605 |
| 储存条件 | 2-8°C |
| 计算化学 | 1.疏水参数计算参考值(XlogP):4.1 2.氢键供体数量:4 3.氢键受体数量:12 4.可旋转化学键数量:3 5.互变异构体数量:339 6.拓扑分子极性表面积:195 7.重原子数量:50 8.表面电荷:0 9.复杂度:1480 10.同位素原子数量:0 11.确定原子立构中心数量:9 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:3 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RTECS号 | KD1925000 |
|---|---|
| 海关编码 | 2941903000 |
|
~92% 
13553-79-2 |
| 文献:Seong, Baik Lin; Han, Moon Hi Chemistry Letters, 1982 , p. 627 - 628 |
|
~% 
13553-79-2 |
| 文献:Journal of the Chemical Society, Chemical Communications, , p. 395 - 396 |
|
~% 
13553-79-2 |
| 文献:Journal of the Chemical Society, Chemical Communications, , p. 395 - 396 |
| 上游产品 3 | |
|---|---|
| 下游产品 2 | |
| 海关编码 | 2941903000 |
|---|