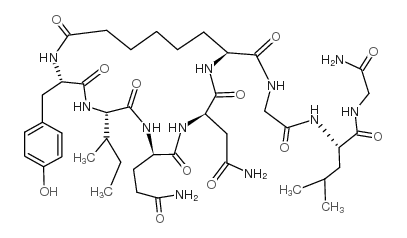
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
RS7580000
-
CHEMICAL NAME :
-
Oxytocin, 1-butyric acid-6-(L-2-aminobutyric acid)-7-glycine-
-
CAS REGISTRY NUMBER :
-
33605-67-3
-
LAST UPDATED :
-
199601
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C42-H65-N11-O12
-
MOLECULAR WEIGHT :
-
916.18
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 ku/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,695,1982 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
17500 units/kg/13W-I
-
TOXIC EFFECTS :
-
Related to Chronic Data - changes in uterine weight
-
REFERENCE :
-
YKYUA6 Yakkyoku. Pharmacy. (Nanzando, 4-1-11, Yushima, Bunkyo-ku, Tokyo, Japan) V.1- 1950- Volume(issue)/page/year: 35,1071,1984 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
27 iu/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 10,41,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
11 iu/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 10,26,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
1100 iu/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 10,26,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
13 iu/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 10,26,1979
|