CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XM7580000
-
CHEMICAL NAME :
-
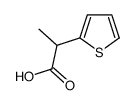
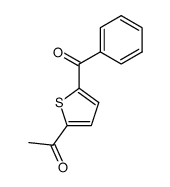
2-Thiopheneacetic acid, 5-benzoyl-alpha-methyl-
-
CAS REGISTRY NUMBER :
-
33005-95-7
-
BEILSTEIN REFERENCE NO. :
-
1380662
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
16
-
MOLECULAR FORMULA :
-
C14-H12-O3-S
-
MOLECULAR WEIGHT :
-
260.32
-
WISWESSER LINE NOTATION :
-
T5SJ BY1&VQ EVR
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
168 mg/kg/14D-I
-
TOXIC EFFECTS :
-
Vascular - shock Lungs, Thorax, or Respiration - other changes Gastrointestinal - ulceration or bleeding from small intestine
-
REFERENCE :
-
MJAUAJ Medical Journal of Australia. (Australasian Medical Pub. Co. Ltd., 71-79 Arundel St., Glebe, N.S.W., Australia) V.1- 1914- Volume(issue)/page/year: 158,647,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
672 mg/kg/7W-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - anorexia (human) Liver - other changes
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,803,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
16 mg/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - wakefulness Behavioral - hallucinations, distorted perceptions Behavioral - excitement
-
REFERENCE :
-
CMAJAX Canadian Medical Association Journal. (Canadian Medical Assoc., POB 8650, Ottawa, ON K1G 0G8, Canada) V.1- 1911- Volume(issue)/page/year: 137,1022,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
257 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Kidney, Ureter, Bladder - inflammation, necrosis, or scarring of bladder
-
REFERENCE :
-
MJAUAJ Medical Journal of Australia. (Australasian Medical Pub. Co. Ltd., 71-79 Arundel St., Glebe, N.S.W., Australia) V.1- 1914- Volume(issue)/page/year: 160,123,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
720 mg/kg/60D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Kidney, Ureter, Bladder - inflammation, necrosis, or scarring of bladder
-
REFERENCE :
-
MJAUAJ Medical Journal of Australia. (Australasian Medical Pub. Co. Ltd., 71-79 Arundel St., Glebe, N.S.W., Australia) V.1- 1914- Volume(issue)/page/year: 160,123,1994
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
181 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 9,715,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
225 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 9,715,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
215 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 15,688,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
690 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - antipsychotic
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 9,715,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
355 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #4159986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
675 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 15,688,1984 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2190 mg/kg/1Y-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes Related to Chronic Data - death Related to Chronic Data - changes in ovarian weight
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,1743,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
male 10 week(s) pre-mating female 2 week(s) pre-mating - 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
JZKEDZ Jitchuken Zenrinsho Kenkyuho. Central Institute for Experimental Animals, Research Reports. (Jikken Dobutsu Chuo Kenkyusho, 1433 Nogawa, Takatsu-ku, Kawasaki 211, Japan) V.1- 1975- Volume(issue)/page/year: 6,195,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
7200 mg/kg
-
SEX/DURATION :
-
male 10 week(s) pre-mating female 2 week(s) pre-mating - 6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
JZKEDZ Jitchuken Zenrinsho Kenkyuho. Central Institute for Experimental Animals, Research Reports. (Jikken Dobutsu Chuo Kenkyusho, 1433 Nogawa, Takatsu-ku, Kawasaki 211, Japan) V.1- 1975- Volume(issue)/page/year: 6,195,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JZKEDZ Jitchuken Zenrinsho Kenkyuho. Central Institute for Experimental Animals, Research Reports. (Jikken Dobutsu Chuo Kenkyusho, 1433 Nogawa, Takatsu-ku, Kawasaki 211, Japan) V.1- 1975- Volume(issue)/page/year: 6,205,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
975 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,1773,1980
|





![[5-(1-methoxyethyl)thiophen-2-yl]-phenylmethanone结构式](https://image.chemsrc.com/caspic/368/143381-62-8.png)
![[5-(1-hydroxyethyl)thiophen-2-yl]-phenylmethanone结构式](https://image.chemsrc.com/caspic/302/143379-91-3.png)