恩替卡韦
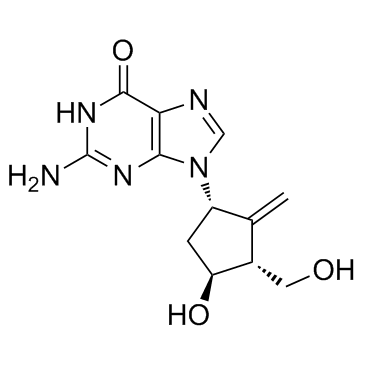
恩替卡韦结构式

|
常用名 | 恩替卡韦 | 英文名 | Entecavir |
|---|---|---|---|---|
| CAS号 | 142217-69-4 | 分子量 | 277.279 | |
| 密度 | 1.8±0.1 g/cm3 | 沸点 | 734.2ºC at 760 mmHg | |
| 分子式 | C12H15N5O3 | 熔点 | 249-252ºC | |
| MSDS | 美版 | 闪点 | 397.9ºC |
恩替卡韦用途Entecavir(SQ 34676; BMS 200475)是有选择且有效地HBV抑制剂。在HepG2细胞中的EC50值为3.75 nM。 |
||||
恩替卡韦作用本品为鸟嘌呤核苷类似物,对乙肝病毒(HBV)多聚酶具有抑制作用。它能够通过磷酸化成为具有活性的三磷酸盐,三磷酸盐在细胞内的半衰期为15小时。通过与HBV多聚酶的天然底物三磷酸脱氧鸟嘌呤核苷竞争,恩替卡韦三磷酸盐能抑制病毒多聚酶(逆转录酶)的所有三种活性:(1)HBV多聚酶的启动;(2)前基因组mRNA逆转录负链的形成;(3)HBV DNA正链的合成。恩替卡韦三磷酸盐对HBV DNA多聚酶的抑制常数(Ki)为0.0012μM。恩替卡韦三磷酸盐对细胞的α、β、δDNA多聚酶和线粒体γDNA多聚酶抑制作用较弱,Ki值为18至于160μM。 |
| 中文名 | 恩替卡韦 |
|---|---|
| 英文名 | entecavir (anhydrous) |
| 中文别名 | ENTECAVIR(一水合物) | 2-氨基-9-((1S,3R,4S)-4-羟基-3-(羟甲基)-2-甲烯基环戊基)-3H-嘌呤-6(9H)-酮 | 9-(4-羟基-3-羟甲基-2-亚甲基环戊-1-基)鸟嘌呤 |
| 英文别名 | 更多 |
| 描述 | Entecavir(SQ 34676; BMS 200475)是有选择且有效地HBV抑制剂。在HepG2细胞中的EC50值为3.75 nM。 |
|---|---|
| 相关类别 | |
| 靶点 |
EC50: 3.75 nM (anti-HBV, HepG2 cell)[2] |
| 体外研究 | BMS-200475对HBV的EC50为3.75 nM。将其掺入HBV的蛋白质引物中,随后抑制逆转录酶的引发步骤。 BMS-200475的抗病毒活性明显低于其他RNA和DNA病毒[1]。与其他脱氧鸟苷类似物(喷昔洛韦,更昔洛韦,lobucavir和阿昔洛韦)或拉米夫定相比,恩替卡韦更容易被磷酸化为其活性代谢物。恩替卡韦的细胞内半衰期为15小时[2]。 |
| 体内研究 | BMS-200475每日口服治疗,剂量范围为0.02至0.5毫克/千克体重,持续1至3个月,可有效降低慢性感染土拨鼠中土拨鼠肝炎病毒(WHV)病毒血症的水平[3]。 |
| 细胞实验 | BMS 200475在磷酸盐缓冲盐水(PBS)中制备,并用含有2%胎牛血清的适当培养基稀释。将HepG2 2.2.15细胞以每孔5×10 5个细胞的密度接种在12孔Biocoat胶原包被的平板上,并保持在融合状态2至3天,然后用1mL掺有BMS 200475的培养基覆盖。 HBV的定量在第10天进行[1]。 |
| 参考文献 |
| 密度 | 1.8±0.1 g/cm3 |
|---|---|
| 沸点 | 734.2ºC at 760 mmHg |
| 熔点 | 249-252ºC |
| 分子式 | C12H15N5O3 |
| 分子量 | 277.279 |
| 闪点 | 397.9ºC |
| 精确质量 | 277.117493 |
| PSA | 130.05000 |
| LogP | -0.96 |
| 外观性状 | 白色至灰白色/黄色结晶粉末 |
| 折射率 | 1.837 |
| 储存条件 | -20°C Freezer |
| 危险品运输编码 | NONH for all modes of transport |
|---|---|
| 海关编码 | 2933990090 |
| 海关编码 | 2933990090 |
|---|---|
| 中文概述 | 2933990090. 其他仅含氮杂原子的杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Formation of covalently closed circular DNA in Hep38.7-Tet cells, a tetracycline inducible hepatitis B virus expression cell line.
Biochem. Biophys. Res. Commun. 452(3) , 315-21, (2014) Hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) plays a central role in chronic HBV infection. However, analysis of the molecular mechanism of cccDNA formation is difficult because of ... |
|
|
Influence of the basal core promoter and precore mutation on replication of hepatitis B virus and antiviral susceptibility of different genotypes.
J. Med. Virol. 87(4) , 601-8, (2015) Mutations in the basal core promoter (BCP) and precore (PC) regions of the hepatitis B virus (HBV) are more common in genotypes B and C than in genotype A, suggesting that these mutations might affect... |
|
|
Decreased liver distribution of entecavir is related to down-regulation of Oat2/Oct1 and up-regulation of Mrp1/2/3/5 in rat liver fibrosis.
Eur. J. Pharm. Sci. 71 , 73-9, (2015) We aimed to elucidate whether entecavir was taken-up into liver by transporters and clarify the possible molecular mechanisms of changes in the distribution of entecavir in rat liver fibrosis.Thioacet... |
| 2-Amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-1,9-dihydro-6H-purin-6-one |
| entecavir (anhydrous) |
| 6H-Purin-6-one, 2-amino-1,9-dihydro-9-((1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl)- |
| 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one |
| 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-1,9-dihydro-6H-purin-6-one |
| 6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]- |
| Baraclude |
| entecavir |
| 9H-purin-6-ol, 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]- |
| UNII-NNU2O4609D |
| 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-9H-purin-6-ol |

 CAS号701278-58-2
CAS号701278-58-2![6-(benzyloxy)-9-((4aR,6S,7aS)-5-methylene-2-phenylhexahydrocyclopenta[d][1,3]dioxin-6-yl)-N-(trimethylsilyl)-9H-purin-2-amine结构式](https://image.chemsrc.com/caspic/430/1351691-93-4.png) CAS号1351691-93-4
CAS号1351691-93-4![9-[(1S,3R,4S)-4-tert-butyldimethylsilyloxy-3-(tert-butyldimethylsilyloxymethyl)-2-methylene-cyclopentyl]-6-chloro-9H-purine-2-carbamic acid tert-butyl ester结构式](https://image.chemsrc.com/caspic/217/1354715-15-3.png) CAS号1354715-15-3
CAS号1354715-15-3![6-chloro-9-[(1S,3R,4S)-4-hydroxy-3-hydroxymethyl-2-methylene-cyclopentyl]-N-(tert-butyloxycarbonyl)-9H-purine-2-carbamic acid tert-butyl ester结构式](https://image.chemsrc.com/caspic/455/1354715-31-3.png) CAS号1354715-31-3
CAS号1354715-31-3
