5-chlorosalicylic acid
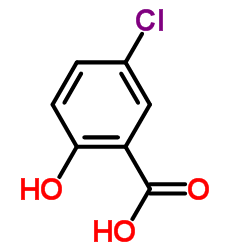
5-chlorosalicylic acid structure
|
Common Name | 5-chlorosalicylic acid | ||
|---|---|---|---|---|
| CAS Number | 321-14-2 | Molecular Weight | 172.566 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 320.5±27.0 °C at 760 mmHg | |
| Molecular Formula | C7H5ClO3 | Melting Point | 171-172 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 147.6±23.7 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Predicting human serum albumin affinity of interleukin-8 (CXCL8) inhibitors by 3D-QSPR approach.
J. Med. Chem. 48 , 2469-79, (2005) A novel class of 2-(R)-phenylpropionamides has been recently reported to inhibit in vitro and in vivo interleukin-8 (CXCL8)-induced biological activities. These CXCL8 inhibitors are derivatives of phenylpropionic nonsteroidal antiinflammatory drugs (NSAIDs), ... |
|
|
Structure-activity relationship of salicylic acid derivatives on inhibition of TNF-α dependent NFκB activity: Implication on anti-inflammatory effect of N-(5-chlorosalicyloyl)phenethylamine against experimental colitis.
Eur. J. Med. Chem. 48 , 36-44, (2012) To develop a more potent NFκB inhibitor from salicylic acid which is known to inhibit activity of NFκB, a transcription factor regulating genes involved in immunity, inflammation and tumorigenesis, derivatives of salicylic acid (SA) where the 5 position, carb... |
|
|
In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II.
Bioorg. Med. Chem. 16 , 9101-5, (2008) The inhibition of two human cytosolic carbonic anhydrase (hCA, EC 4.2.1.1) isozymes, hCA I and II, with a series of salicylic acid derivatives was investigated by using the esterase method with 4-nitrophenyl acetate as substrate. IC(50) values for sulfasalazi... |
|
|
Guest-controlling effects on ER behaviors of beta-cyclodextrin polymer.
J. Colloid. Interface Sci. 289(1) , 56-62, (2005) An effective and novel approach to obtaining electrorheological particles with high performance through the formation of host-guest complexes has been achieved. The significant preponderance of the host-guest complex formation is that the host structure can b... |
|
|
Genotoxicity of ochratoxin A and structurally related compounds in Escherichia coli strains: studies on their mode of action.
IARC Sci. Publ. (115) , 261-6, (1991) Ochratoxin A, ochratoxin alpha (its major metabolite in rodents) and seven structurally related substances were assayed for SOS DNA repair inducing activity in Escherichia coli PQ37 strain. At a concentration range of 0.1-4 mM, ochratoxin A, chloroxine, 5-chl... |
|
|
Mutagenic activity of 2-chloro-4-nitroaniline and 5-chlorosalicylic acid in Salmonella typhimurium: two possible metabolites of niclosamide.
Mutat. Res. 264(3) , 139-45, (1991) Niclosamide is an anti-helminthic drug susceptible to being metabolized into a bacterial mutagen by the action of enzymes present in the S9 activation mixture. Additional results from genotoxic studies in rodents and humans suggest that the drug is absorbed f... |
|
|
Effect of biological confinement on the photophysics and dynamics of a proton-transfer phototautomer: an exploration of excitation and emission wavelength-dependent photophysics of the protein-bound drug.
Phys. Chem. Chem. Phys. 14(35) , 12182-92, (2012) The present work demonstrates the effect of biological confinement on the photophysics and dynamics of a bio-active drug molecule viz., 5-chlorosalicylic acid (5ClSA). 5ClSA is a potential candidate exhibiting Excited-State Intramolecular Proton Transfer (ESI... |
|
|
Comparative gastric ulcerogenic effects of meseclazone, 5-chlorosalicylic acid and other nonsteroidal anti-inflammatory drugs following acute and repeated oral administration to rats.
Toxicol. Appl. Pharmacol. 52(3) , 454-61, (1980)
|
|
|
New bacterial pathway for 4- and 5-chlorosalicylate degradation via 4-chlorocatechol and maleylacetate in Pseudomonas sp. strain MT1.
J. Bacteriol. 185(23) , 6790-800, (2003) Pseudomonas sp. strain MT1 is capable of degrading 4- and 5-chlorosalicylates via 4-chlorocatechol, 3-chloromuconate, and maleylacetate by a novel pathway. 3-Chloromuconate is transformed by muconate cycloisomerase of MT1 into protoanemonin, a dominant reacti... |
|
|
Two angular dioxygenases contribute to the metabolic versatility of dibenzofuran-degrading Rhodococcus sp. strain HA01.
Appl. Environ. Microbiol. 74(12) , 3812-22, (2008) Rhodococcus sp. strain HA01, isolated through its ability to utilize dibenzofuran (DBF) as the sole carbon and energy source, was also capable, albeit with low activity, of transforming dibenzo-p-dioxin (DD). This strain could also transform 3-chlorodibenzofu... |