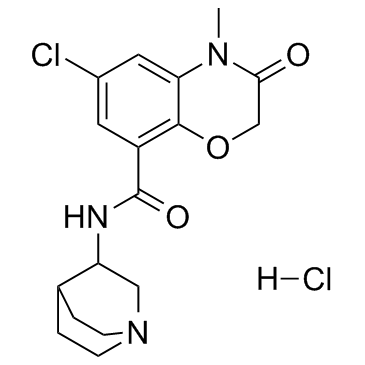
123040-16-4
| Name | Azasetron hydrochloride |
|---|---|
| Synonyms |
Azasetron HCl
UNII:2BSS7XL60S AZASETRON Azasetron (hydrochloride) MFCD00209913 Y-25130 Hydrochloride N-1-AZABICYCLO[2.2.2]OCT-3-YL-6-CHLORO-3,4-DIHYDRO-4-METHYL-3-OXO-2H-1,4-BENZOXAZINE-8-CARBOXAMIDE Serotone 2H-1,4-BENZOXAZINE-8-CARBOXAMIDE,N-1-AZABICYCLO[2.2.2]OCT-3-YL-6-CHLORO-3,4-DIHYDRO-4-METHYL-3-OXO-,HYDROCHLORIDE (1:1) N-(1-Azabicyclo[2.2.2]oct-3-yl)-6-chloro-4-methyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide hydrochloride (1:1) INTERMEDIATE OF AZASETRON HCL 2H-1,4-BENZOXAZINE-8-CARBOXAMIDE, 3,4-DIHYDRO-N-1-AZABICYCLO(2.2.2)OCT-3-YL-6-C Azasetron hydrochloride 2H-1,4-Benzoxazine-8-carboxamide, N-1-azabicyclo[2.2.2]oct-3-yl-6-chloro-3,4-dihydro-4-methyl-3-oxo-, hydrochloride (1:1) N-(1-AZABICYCLO[2.2.2]OCT-3-YL)-6-CHLORO-4-METHYL-3-OXO-3,4-DIHYDRO-2H-1,4-BENZOXAZINE-8-CARBOXAMIDE HYDROCHLORIDE Azasetronhydrochloride |
| Description | Azasetron HCl is a selective 5-HT3 receptor antagonist with IC50 of 0.33 nM used in the management of nausea and vomiting induced by cancer chemotherapy. Target: 5-HT3 ReceptorAzasetron Hydrochloride is a 5-HT3 receptor antagonist which is used as an anti-emetic.Azasetron inhibited the specific binding of [3H]quipazine to 5-HT3 receptors at the synaptic membranes of the rat cerebral cortex with a Ki value of 2.9 nM. Azasetron showed low affinity for histamine H1 receptors (IC50 = 4.4 microM) but it could not reveal any affinities for the other receptors (5-HT1A, 5-HT2, dopamine D1, dopamine D2, alpha 1-adrenoceptor, alpha 2-adrenoceptor, muscarine and benzodiazepine) even at a 10 microM concentration [1]. Azasetron (0.1-1.0 mg/kg) dose-dependently prolonged the latency to the first vomiting and decreased the number of vomitings induced by cisplatin in dogs. Azasetron is an orally active antiemetic compound against cisplatin and doxorubicin/cyclophosphamide-induced emeses; and its the antiemetic potency is similar to those of granisetron and ondansetron, but superior to those of metoclopramide and domperidone [2]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 558ºC at 760 mmHg |
|---|---|
| Molecular Formula | C17H21Cl2N3O3 |
| Molecular Weight | 386.273 |
| Flash Point | 291.2ºC |
| Exact Mass | 385.096008 |
| PSA | 61.88000 |
| LogP | 2.71500 |
| Storage condition | Desiccate at -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S22-S24/25 |
|---|---|
| WGK Germany | 3 |
|
~% 
123040-16-4 |
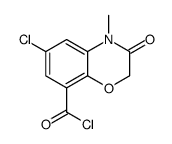
| Literature: US4892872 A1, ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |