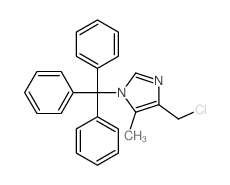
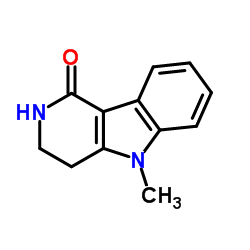
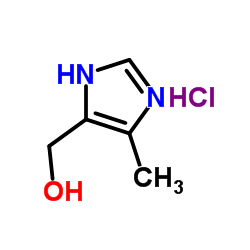
122852-42-0
| Name | alosetron |
|---|---|
| Synonyms |
UNII-13Z9HTH115
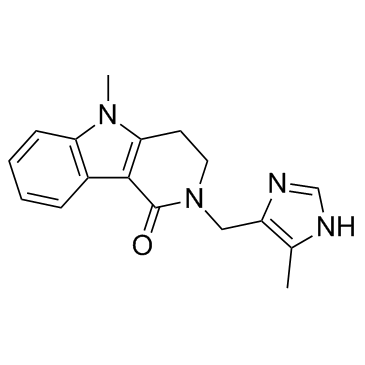
5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one Alosetron HCl |
| Description | Alosetron is a Serotonin 5HT3-receptor antagonist that is used in treatment of irritable bowel syndrome.IC50 Value: Target: 5-HT ReceptorAlosetron has an antagonist action on the 5-HT3 receptors of the enteric nervous system of the gastrointestinal tract. While being a 5-HT3 antagonist like ondansetron, it is not classified or approved as an antiemetic. Since stimulation of 5-HT3 receptors is positively correlated with gastrointestinal motility, alosetron's 5-HT3 antagonism slows the movement of fecal matter through the large intestine, increasing the extent to which water is absorbed, and decreasing the moisture and volume of the remaining waste products. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.34 g/cm3 |
|---|---|
| Boiling Point | 648.1ºC at 760 mmHg |
| Molecular Formula | C17H18N4O |
| Molecular Weight | 294.35100 |
| Flash Point | 345.8ºC |
| Exact Mass | 294.14800 |
| PSA | 53.92000 |
| LogP | 2.34620 |
| Vapour Pressure | 1.1E-16mmHg at 25°C |
| Index of Refraction | 1.706 |
| Storage condition | 2-8℃ |
|
~% 
122852-42-0 |
| Literature: US5221687 A1, ; |
|
~28% 
122852-42-0 |
| Literature: AUSPEX PHARMACEUTICALS, INC. Patent: US2010/113478 A1, 2010 ; Location in patent: Page/Page column 13 ; |
|
~63% 
122852-42-0 |
| Literature: ORCHID CHEMICALS and PHARMACEUTICALS LIMITED Patent: US2012/178937 A1, 2012 ; Location in patent: Page/Page column 3 ; |
|
~% 
122852-42-0 |
| Literature: US5196534 A1, ; US 5196534 A |
| Precursor 5 | |
|---|---|
| DownStream 0 | |