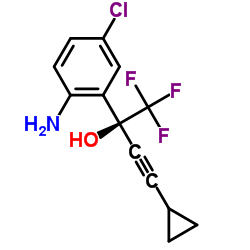
154598-52-4
| Name | efavirenz |
|---|---|
| Synonyms |
Stocrin
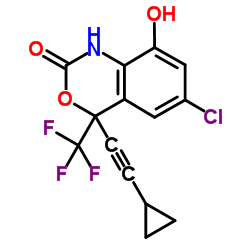
EFAVIRNEZ Efavirenz (200 mg) (S)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-2H-3,1-benzoxazin-2-one (4S)-6-Chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-4H-3,1-benzoxazin-2-ol (4S)-6-Chloro-4-(cyclopropylethynyl)-4-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-2-one 4H-3,1-Benzoxazin-2-ol, 6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-, (4S)- DMP 266 SUSTIVA MDP-266 Efavirenz 2H-3,1-Benzoxazin-2-one, 6-chloro-4-(2-cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-, (4S)- |
| Description | Efavirenz is a potent inhibitor of the wild-type HIV-1 reverse transcriptase with a Ki of 2.93 nM and exhibits an IC95 of 1.5 nM for the inhibition of HIV-1 replicative spread in cell culture. |
|---|---|
| Related Catalog | |
| Target |
Ki: 2.93 nM (HIV-1 RT)[1] |
| In Vitro | Efavirenz (L-743726) is found to be capable of inhibiting, with 95% inhibitory concentrations of ≤ 1.5μM, a panel of nonnucleoside reverse transcriptase (RT) inhibitors (NNRTIs)-resistant mutant viruses, each of which expresses a single RT amino acid substitution. Efavirenz is also tested for its activity against a variety of polymerase enzymes and is found to be inactive (IC50>300μM). Efavirenz effectively inhibits several wild-type T-lymphoid cell line-adapted variants. Identical activity (IC95, 1.5 to 3.0 nM) is seen with wild-type primary isolates of the virus in both primary lymphoid and monocytoid cell cultures. Efavirenz also effectively inhibits HIV-1 variants that expressed RT amino acid substitutions which confer the loss of susceptibility to other NNRTIs. For purposes of comparison[1]. Efavirenz is a non-nucleoside analog reverse transcriptase inhibitor (NNRTI) with IC50 of 60 nM[2]. Efavirenz inhibits synthesis using an RNA PPT-primed substrate with an IC50 of 17 nM[3]. |
| In Vivo | After i.v. administration, Efavirenz (L-743726) is cleared rapidly from rats, but it is cleared considerably more slowly from monkeys. The large volume of distribution (two to four times the amount of body water) in both species indicates extensive tissue binding. The oral bioavailability in rats is 16%. In monkeys, the half-life of Efavirenz after administration of a 1 mg/kg i.v. dose exceeded 2.5 h. Efavirenz is well absorbed orally. Administration to monkeys of oral doses as fine suspensions in 0.5% aqueous methylcellulose yields consistently high levels in plasma. A 2.0 mg/kg dose produces peak levels of 0.5μM at approximately 3.0 h. The absolute bioavailability is estimated to be 42%. A 10 mg/kg dose yields a peak level in plasma of 3.22 μM. A 10 mg/kg oral dose given to a single chimpanzee gave concentrations in plasma of 4.12, 2.95, and 2.69 μM at 2, 8, and 24 h after dosing, respectively[1]. |
| Kinase Assay | Recombinant RT enzymes are expressed, purified, and assessed for inhibition by Efavirenz (L-743726). Ki and Kii values are determined for each enzyme tested. The wild-type RT exhibited exclusively noncompetitive inhibition kinetics (data not shown), and, therefore, the Ki and Kii values are identical. Pure noncompetitive inhibition is not assumed for the mutant enzymes, and, hence, the values of both Ki and Kii are obtained from the linear mixed-type inhibition equation. The two- to threefold differences between the Ki and Kii values probably reflect a small contribution of competitive inhibition with the mutant RTs[1]. |
| Animal Admin | Mice[1] Studies are performed in rats, rhesus monkeys, and a single chimpanzee. For analyses of the drug given to rats intravenously (i.v.), a group (n=4 or 5) of fasted male Sprague-Dawley rats (weight, 250 to 450 g) receive a bolus (at a volume of 1mL/kg of body weight) of Efavirenz in DMSO via a cannula implanted in the right jugular vein. For oral studies, rats are dosed by gavage by using a suspension of Efavirenz prepared in 0.5% aqueous methylcellulose. Similarly, four monkeys receive either an i.v. bolus of the compound in DMSO via the saphenous vein at a volume of 0.1 mL/kg or are administered the compound orally in suspension by using a nasogastric tube. Monkeys are fasted for 18 h prior to dosing. One nonanesthetized, nonfasted male chimpanzee (weight, approximately 60 kg) is dosed orally by voluntary ingestion by using an aqueous suspension of the compound. In all studies, heparinized blood is obtained at appropriate times. Plasma is separated immediately by centrifugation and is stored at -20°C until analysis. Plasma samples are extracted with methylene chloride; this is followed by analysis by high-performance liquid chromatography. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 422.7±55.0 °C at 760 mmHg |
| Melting Point | 139-141ºC |
| Molecular Formula | C14H9ClF3NO2 |
| Molecular Weight | 315.675 |
| Flash Point | 209.4±31.5 °C |
| Exact Mass | 315.027405 |
| PSA | 38.33000 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H410 |
| Precautionary Statements | P273 |
| Hazard Codes | N |
| Risk Phrases | 50 |
| Safety Phrases | 61 |
| RIDADR | UN3082 - class 9 - PG 3 - DOT NA1993 - Environmentally hazardous substances, liquid, n.o.s. HI: all (not BR) |
| RTECS | DM3440000 |
| HS Code | 2942000000 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2942000000 |
|---|


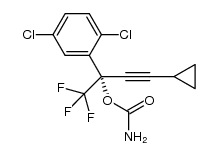
![[4-Chloro-2-[(1S)-3-cyclopropyl-1-hydroxy-1-(trifluoromethyl)-2-propynyl)phenyl]carbamic Acid Methyl Ester structure](https://image.chemsrc.com/caspic/257/211563-40-5.png)
![(S)-2-(5-chloro-2-((1S,4R)-4,7,7-trimethyl-3-oxo-2-oxabicyclo[2.2.1]heptane-1-carboxamido)phenyl)-4-cyclopropyl-1,1,1-trifluorobut-3-yn-2-yl 1-chloroethyl carbonate structure](https://image.chemsrc.com/caspic/263/1262030-22-7.png)