| Description |
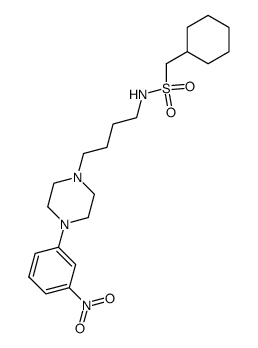
Naluzotan is a novel, potent, and selective amidosulfonamide 5-HT1A agonist with IC50 and Ki of appr 20 nM and 5.1 nM, used for the treatment of anxiety and depression; Also a weak hERG K+ channel blocker, with IC50 of 3800 nM.
|
| Related Catalog |
|
| Target |
IC50: appr 20 nM (5-HT1A)[2], 3800 nM (hERG K+ channel)[1] Ki: 5.1 nM (5-HT1A)[1]
|
| In Vitro |
Naluzotan behaves as a full agonist in an in vitro cell-based functional assay with an EC50 of 20 nM. Naluzotan has significant affinity is the guinea pig sigma receptor (Ki = 100 nM), but does not inhibit cytochrome P450 isoforms (CYP) 1A2, 2C9, 2C19, 2D6, and 3A4[1].
|
| In Vivo |
In rats Naluzotan shows 11% oral bioavailability with a serum t1/2 of 2−3.5 h when administrated po, attaining a Cmax level of 24 ± 13 ng/mL (3 mg/kg, po). Naluzotan shows significant brain penetration, achieving a brain:serum concentration ratio of approximately 0.5 in the rat at 1 h following either intravenous or oral administration and reaching brain concentration approximately equivalent to that of buspirone. In dogs the pharmacokinetic profile of naluzotan shows 16% oral bioavailability, a serum t1/2 of 1.1 h po, and a Cmax level of 174 ± 141 ng/mL (3 mg/kg, po)[1]. PRX-00023 (0.01-0.05 mg/kg, i.p.) significantly reduces USV rates, but done of these doses produce sedation in rats[2].
|
| Animal Admin |
PRX-00023 and buspirone are used in the assay. Drugs are dissolved in saline vehicle prior to injections. Each pup is injected in the intraperitoneal space (i.p.) with one of several doses of PRX-00023 (0.01, 0.03, 0.05, 0.1, 0.3, 1.0, and 3.0 mg/kg in saline, for a total volume of 0.1 mg/kg). Within each litter two littermates, though occasionally one, receive the same dose of a compound. Because of the distribution of pups in litters used, no Random line pups are tested with PRX-00023 at 3.0 mg/kg for comparison to vehicle.
|
| References |
[1]. Becker OM, et al. An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the treatment of anxiety and depression. J Med Chem. 2006 Jun 1;49(11):3116-35. [2]. Brunelli SA, et al. PRX-00023, a selective serotonin 1A receptor agonist, reduces ultrasonic vocalizations in infant rats bred for high infantile anxiety. Pharmacol Biochem Behav. 2009 Nov;94(1):8-15.
|