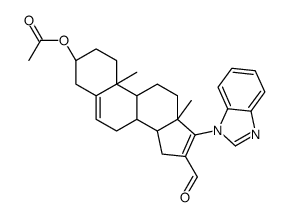
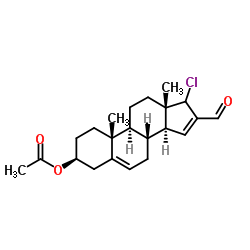
851983-85-2
| Name | (3S,8R,9S,10R,13S,14S)-17-(benzimidazol-1-yl)-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-ol |
|---|---|
| Synonyms |
Androsta-5,16-dien-3-ol, 17-(1H-benzimidazol-1-yl)-, (3β)-
TOK-001 VN/124-1 (3β)-17-(1H-Benzimidazol-1-yl)androsta-5,16-dien-3-ol (3S,8R,9S,10R,13S,14S)-17-(1H-benzo[d]imidazol-1-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol VN/124 Galeterone |
| Description | TOK-001 is a multifunctional antiandrogen and CYP17 inhibitor (IC50=47 nM) in castration resistant prostate cancer (CRPC). |
|---|---|
| Related Catalog | |
| Target |
IC50: 47 nM (CYP17)[1] |
| In Vitro | TOK-001 affords strong CYP17 lyase inhibition, with IC50 of 47 nM[1]. TOK-001 is both a CYP17A1 inhibitor and androgen receptor antagonist and the similarity of these binding modes is likely the reason for this dual mechanism of action.This CYP17A1 binds abiraterone and TOK-001 with absorbance decreases at 402 nm and increases at 424 nm, consistent with nitrogen binding to the heme iron (type II interaction) with Kd of <100 nM[2]. When LNCaP cells are cultured in medium supplemented with charcoal-stripped serum (CSS, T<1 nM) followed by treatment with increasing concentrations of TOK-001, the steady-state levels of AR protein are markedly decreased (up to 84%, 15 μM TOK-001). In LAPC-4 cells, abiraterone alcohol reduced AR expression to a greater extent than TOK-001 at concentrations greater than or equal to 1 μM. When LNCaP cells are treated with 20 μM TOK-001 for 24 h, AR mRNA levels are reduced by 38%[3]. |
| In Vivo | Mice inoculated with LAPC-4 tumors are treated subcutaneously with 0.15 mmol/kg of TOK-001 twice daily. Mice treated with TOK-001 have smaller average tumor volume on day 31 when compared to control (p= 0.0001). TOK-001 treatment also significantly reduces the growth rate of tumor growth compared to control (p<0.0001). Upon excision, final tumor weights are also significantly reduced in animals treated with TOK-001 compared to animals treated with control, and castration (p<0.05)[1]. |
| Kinase Assay | Progesterone 17α-hydroxylation is evaluated using a modified HPLC method with UV-detection. CYP17A1 (50 pmol) and rat NADPH-cytochrome P450 reductase 1:4 are mixed, incubated on ice (20 minutes), and added to buffer (50 mM Tris, pH 7.4 and 5 mM MgCl2) containing progesterone (0-50 μM) to a total volume of 500 μL. Phosphatidylcholine (25 μg) is included for side-by-side kinetic comparisons with the full-length enzyme. For IC50 determinations, inhibitors concentrations are 0-1500 nM for abiraterone and 0-3000 nM for TOK-001 (Shanghai Haoyuan Chemexpress Co., Shanghai, China). After warming (37°C, 3 minutes), reactions are initiated by NADPH addition (20 μL 25 mM), incubated for 10 minutes (37°C), and quenched with 20% trichloroacetic acid (300 μL) and placed on ice. The 17α-hydroxyprogesterone metabolite is identified by UV detection at 248 nm following HPLC separation and coeluted with authentic standards. The HPLC mobile phase is 40% acetonitrile, 60% water with 1% acetic acid and run at 1 mL/min (Phenomenex, Luna 5 μ, C18, 50×4.6 mm)[2]. |
| Cell Assay | LNCaP cells are seeded in 24-well plates at 70% confluence a day before transfections. Cells then are transfected with plasmid DNA (0.5 μg/well) carrying pIR-AR 5′UTR-Luc (5′UTR; test) or pIRES-Luc (IRES, control) for 6 h in serum-free and antibiotic-free conditions. Transcription of both luciferase reporter genes is under the control of the CMV promoter. Lipofectamine 2000 reagent is used in all transfections according to the manufacturer's instructions. Cells are then treated with doses of TOK-001 (0, 10, and 20 μM) in 5% FBS/T-Medium. Luciferase reporter gene activities are measured using Luciferase Assay System from Promega at 36 h post treatment using a BMG Labtech microplate reader. Relative luciferase units are normalized to total protein and then normalized to vector control (pIR-AR 5′UTR-Luc) and the result is presented as luciferase activity. For cell proliferation studies, LNCaP cells in 96-well plate are seeded 24 h prior to drug treatment and then treated with control (mock), TOK-001 (10 μM), or abiraterone alcohol (10 μM) in 5% FBS/T-medium for 72 h. Cell proliferation is determined using MTS[3]. |
| Animal Admin | Mice[1] Mice inoculated with LAPC-4 tumors are treated subcutaneously with 0.15 mmol/kg of TOK-001 twice daily. Mice treated with TOK-001 have smaller average tumor volume on day 31 when compared to control (p=0.0001). TOK-001 treatment also significantly reduced the growth rate of tumor growth compared to control (p<0.0001). Upon excision, final tumor weights are also significantly reduced in animals treated with TOK-001 compared to animals treated with control, and castration (p<0.05). |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 564.5±60.0 °C at 760 mmHg |
| Melting Point | 189-190℃ |
| Molecular Formula | C26H32N2O |
| Molecular Weight | 388.545 |
| Flash Point | 295.2±32.9 °C |
| Exact Mass | 388.251465 |
| PSA | 38.05000 |
| LogP | 6.28 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.693 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
~91% 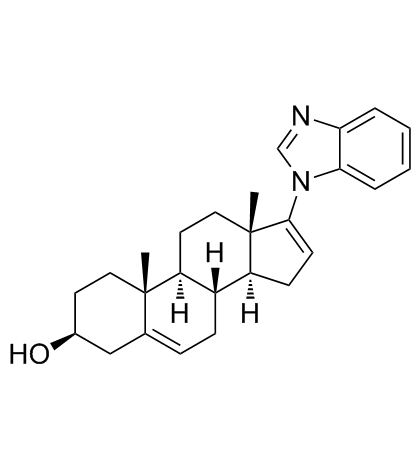
851983-85-2 |
| Literature: UNIVERSITY OF MARYLAND Patent: WO2006/93993 A1, 2006 ; Location in patent: Page/Page column 27; 28; 44 ; WO 2006/093993 A1 |
|
~% 
851983-85-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 48, # 8 p. 2972 - 2984 |
|
~% 
851983-85-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 48, # 8 p. 2972 - 2984 |
|
~% 
851983-85-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 48, # 8 p. 2972 - 2984 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |