82419-36-1
| Name | ofloxacin |
|---|---|
| Synonyms |
7H-1,4-Oxazino[2,3,4-ij]quinoline-6-carboxylic acid, 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-
9-Fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Exocin Floxal (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic Acid 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-1,4oxazino2,3,4-ijquinoline-6-carboxylic acid Visren Floxil Ofloxacin 9-Fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Floxin Oflocin hoe280 Flobacin 9-fluoro-3-methyl-10-(4-methylpiperazino)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid T666 1A M BN EV KO&&TJ DVQ HF M1 I- AT6N DNTJ D1 TARIVID pt01 oflx Oflox MFCD00226105 (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7- -oxo-7H-pyrido(1,2,3-de)- -1,4-benzoxazine-6-carboxylic acid OCUFLOX |
| Description | Ofloxacin is a fluoroquinolone whose primary mechanism of action is inhibition of bacterial DNA gyrase.Target: DNA gyrase Ofloxacin is a fluoroquinolone whose primary mechanism of action is inhibition of bacterial DNA gyrase. In vitro it has a broad spectrum of activity against aerobic Gram-negative and Gram-positive bacteria, although it is poorly active against anaerobes [1]. Ofloxacin, like other 4-quinolones, is unusual among front line drugs available to treat bacterial infections since it affects bacterial DNA synthesis, rather than cell wall or protein synthesis [2].Ofloxacin (20 mg/kg), norfloxacin (40 mg/kg), pefloxacin mesylate dihydrate (40 mg/kg)and ciprofloxacin (50 mg/kg) were administered by gavage twice daily for three consecutive weeks. 6 weeks after treatment, the test animals were euthanised and Achilles tendon specimens were collected. A computer monitored tensile testing machine was utilised for biomechanical testing. The mean elastic modulus of the control group was significantly higher than that of the norfloxacin and pefloxacin groups (p<0.05 and p<0.01, respectively). The mean yield force (YF) of the control group was significantly higher than those of ciprofloxacin, norfloxacin and pefloxacin groups (p<0.001, p<0.05 and p<0.01, respectively). The mean ultimate tensile force (UTF) of the control group was significantly higher than of the ciprofloxacin, norfloxacin, and pefloxacin groups (p<0.001, p<0.05 and p<0.01, respectively). Hyaline degeneration and fibre disarrangement were observed in the tendons of the ciprofloxacin, pefloxacin, and ofloxacin treated-groups, whereas myxomatous degeneration was observed only in the ciprofloxacin and pefloxacin groups [3].Clinical indications: Bacterial infection; Bacterial respiratory tract infection; Bacterial urinary tract infection Toxicity: tendinopathy; hepatotoxicity; dysglycemia |
|---|---|
| Related Catalog | |
| References |
[2]. Smith JT, et al. Ofloxacin, a bactericidal antibacterial. Chemotherapy. 1991;37 Suppl 1:2-13. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 571.5±50.0 °C at 760 mmHg |
| Melting Point | 270-2750C |
| Molecular Formula | C18H20FN3O4 |
| Molecular Weight | 361.367 |
| Flash Point | 299.4±30.1 °C |
| Exact Mass | 361.143799 |
| PSA | 75.01000 |
| LogP | 0.84 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.670 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn, Xi |
| Risk Phrases | 22-42/43-68-36/37/38 |
| Safety Phrases | S26-S36/37/39-S24/25-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UU8815550 |
| HS Code | 2934999090 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
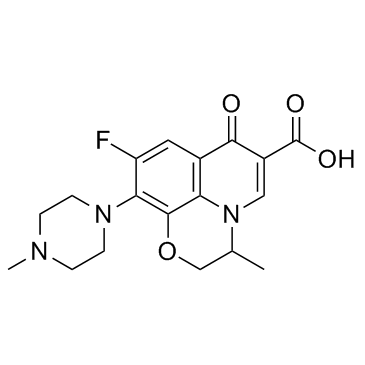

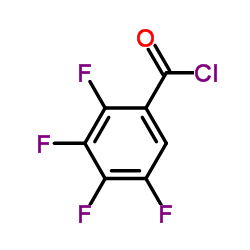
![diethyl [(-)-7,8-difluoro-3-methyl-2,3-dihydro-4H-[1,4]benzoxazin-4-yl]methylenemalonate structure](https://www.chemsrc.com/caspic/261/86760-99-8.png)
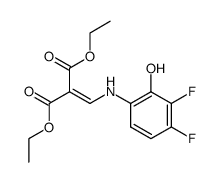
![diethyl [3,4-difluoro-2-(2-hydroxypropyloxy)anilinyl]methylenemalonate structure](https://www.chemsrc.com/caspic/229/124409-86-5.png)