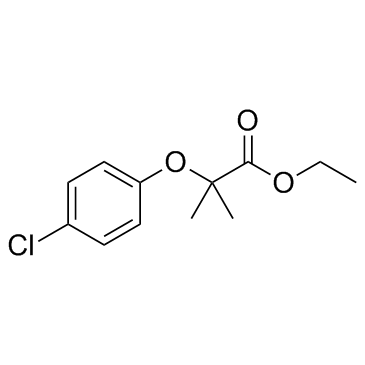
637-07-0
| Name | clofibrate |
|---|---|
| Synonyms |
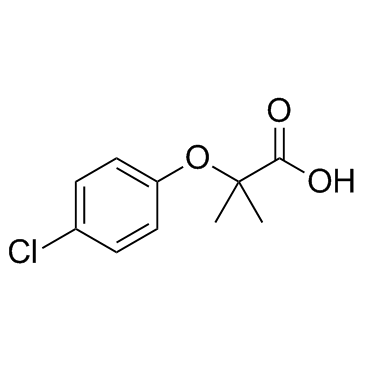
ethyl 2-[(4-chlorophenyl)oxy]-2-methylpropanoate
MFCD00000615 Ethyl α-(p-chlorophenoxy)isobutyrate Ethyl p-chlorophenoxyisobutyrate Ethyl α-(4-chlorophenoxy)isobutyrate Clofipront Clofibric Acid, Ethyl Ester 2-(p-chlorophenoxy)isobutyric acid ethyl ester Ethyl α-(4-chlorophenoxy)-α-methylpropionate clofibrate Atromid Anparton Clofibrato Chlorfenisate Ethyl 2-(4-chlorophenoxy)-2-methylpropanoate Ethyl α-(p-chlorophenoxy)-α-methylpropionate Sklerolip Arterioflexin Antilipid Fibralem ethyl p-chlorophenoxy-isobutyrate Angiokapsul Regelan N EINECS 211-277-4 Isobutyric acid, α-(p-chlorophenoxy)-, ethyl ester Ethyl 2-(4-chlorophenoxy)isobutyrate clofibric acid Ethyl 2-(p-chlorophenoxy)-2-methylpropionate Atromid-S Propanoic acid, 2-(4-chlorophenoxy)-2-methyl-, ethyl ester Regelan Clofibratum Ethyl 2-(4-chlorophenoxy)-2-methylpropionate |
| Description | Clofibrate is an agonist of PPAR, with EC50s of 50 μM, ∼500 μM for murine PPARα and PPARγ, and 55 μM, ∼500 μM for human PPARα and PPARγ, respectively. |
|---|---|
| Related Catalog | |
| Target |
PPARα:50 μM (EC50) PPARγ:500 μM (EC50) |
| In Vitro | Clofibrate is a PPAR agonist, with E50s of 50 μM, ∼500 μM for murine PPARα and PPARγ, and 55 μM, ∼500 μM for human PPARα and PPARγ, respectively[1]. Clofibrate (0.5, 1, 2 mM) increases FABP1 expression in two fatty acid (FA)-treated rat hepatoma cells. Clofibrate lowers ROS levels after early treatment, much more than late treatment in FA-treated cells[2]. |
| In Vivo | Clofibrate (0.5%) up-regulates serum concentrations and hepatic expression of FGF21 in fetuses, with a return to basal levels after Clofibrate administration withdrawal. Clofibrate administration-offspring have significantly higher expression of thermogenic genes (Ucp1, Cidea, Ppara Ppargc1a, Cpt1b) and UCP1 protein levels in response to HFD in inguinal fat, but not in retroperitoneal (combined with perirenal) or epididymal fat[3]. |
| Cell Assay | Cells are seeded at a density of 2.5 × 104 cells/well (for WST-1, intracellular lipid droplet quantification and dichlorofluorescein (DCF) assay, 96-well plates) and 1 × 105 cells/well (for Nile Red Staining, 12-well plates) in MEM/EBSS medium and incubated overnight for adherence. The next day cell culture medium is replaced with freshly prepared medium containing the fatty acid mixture oleate:palmitate (2:1) in presence of 3% fatty-acid-free bovine serum albumin. Cells are treated with 0, 0.5, 1, 2, and 3 mM fatty acid (FA) mixture for 24 and 48 hr at 37°C in a humidified incubator in an atmosphere of 95% air and 5% CO2. Clofibrate is used to increase levels of FABP1 in treated cell cultures. Clofibrate (500 μM) is dissolved in DMSOand later added to the medium (DMSO < 0.1% v/v in final volume). Control cells are incubated with DMSO alone. Four different cell treatments includ 1-day FA treatment, 2-day FA treatment, early clofibrate intervention and late clofibrate intervention[1]. |
| Animal Admin | Female and male C57BL/6JNarl mice are used for breeding. Females with parity from 1 to 5 are used. Pregnant females are fed either a control (C) or experimental (CF) diet from breeding to parturition. The C diet is based on an AIN-93M diet with a slight modification to contain 21 kcal% fat from soybean oil, whereas the CF diet is the C diet with addition of 0.5% clofibrate. Pregnancy is dated by the presence of a vaginal plug (defined as pregnancy day 1). After spontaneous parturition (pregnancy day 19.5 ± 0.5), all littermates are uniformly nursed by dams fed the C diet for 3 wk, with litter sizes adjusted to 8-10, weaned onto a nonpurified standard diet for 4 wk, and then switched to a HFD (51 kcal% fat, butter-based) for 5 wk. In this study, only male offspring are used and 2 groups of offspring are designated, according to their mother's diet (C or CF). All mice are kept in a room maintained at 23 ± 2°C, with a controlled 12-h-light:-dark cycle with ad libitum to feed and drinking water. Body weight and feed intake are recorded weekly[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 274.8±0.0 °C at 760 mmHg |
| Molecular Formula | C12H15ClO3 |
| Molecular Weight | 242.699 |
| Flash Point | 115.1±19.9 °C |
| Exact Mass | 242.070969 |
| PSA | 35.53000 |
| LogP | 3.32 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.505 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R40 |
| Safety Phrases | S26-S36/37/39-S61-S45-S36/37 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| RTECS | UE9480000 |
| HS Code | 2918990090 |
| Precursor 10 | |
|---|---|
| DownStream 5 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |