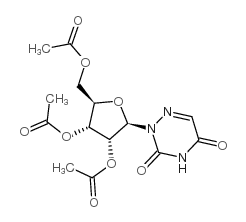
2169-64-4
| Name | 6-azauridine 2',3',5'-triacetate |
|---|---|
| Synonyms |
EINECS 218-515-6
6-AZURD-TA 6-azauridine-2',3',5'-tri-O-acetate triacetyl-6-azauridine ta-azur cb304 2',3',5'-TRI-O-ACETYL-6-AZAURIDINE AZARIBINE 2',3',5'-triacetyl-6-azauridine |
| Description | Azaribine (2',3',5'-Tri-O-acetyl-6-azauridine) is a potent orotidine monophosphate decarboxylase (OMPD) inhibitor. Azaribine is an antiviral inhibitor of several RNA viruses and inhibits viral genome replication and gene transcription. Azaribine shows broad-spectrum antiviral activity (EC50=3.80 nM-1.73 μM against influenza A and B viruses; EC50=1.62 μM against ZIKV Paraiba). Azaribine, a triacetate salt of Azauridine, has the potential for psoriasis research[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Azaribine (2',3',5'-Tri-O-acetyl-6-azauridine; 0-2 μM; 48 小时) 对MDCK细胞具有细胞毒性 (MTT: CC50=19.66 μM)[1]。 Azaribine 对 BIRFLU 增殖具有强效抑制作用 (MDCK 细胞: EC50=0.29 μM;A549 细胞: EC50=0.55 μM) [1]。 Azaribine 在 MDCK 细胞后处理期间显示出对季节性 H1N1 和 H3N2 IAV 和 IBV 的抑制作用,EC50 分别为 0.60 μM、0.77 μM、0.80 μM[1]。 Azaribine 在 16HBE 细胞的后处理过程中具有抗 NC H1N1 作用,EC50 为 1.58 μM[1]。 Cell Cytotoxicity Assay[1] Cell Line: MDCK cells Concentration: 0-2 μM Incubation Time: 48 h Result: Had cytotoxicity on MDCK cells (CC50=19.66 μM). |
| References |
| Density | 1.6g/cm3 |
|---|---|
| Melting Point | 99-101ºC(lit.) |
| Molecular Formula | C14H17N3O9 |
| Molecular Weight | 371.29900 |
| Exact Mass | 371.09600 |
| PSA | 155.88000 |
| Index of Refraction | 1.621 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S22-S24/25 |
|---|---|
| WGK Germany | 2 |
| RTECS | XY8577000 |