13850-16-3
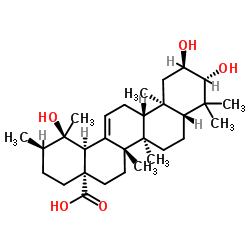
| Name | (1R,2R,4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-1,10,11-trihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid |
|---|---|
| Synonyms |
Urs-12-en-28-oic acid, 2,3,19-trihydroxy-, (2α,3β)-
Jacarandic acid tomentic acid Tormentic acid (2α,3β)-2,3,19-Trihydroxyurs-12-en-28-oic acid Euscaphic acid 2,3,19-trihydroxyurs-12-en-28-oic acid |
| Description | Tormentic acid, a triterpene isolated from Rosa rugosa, exerts anti-inflammatory, antihyperlipidemic, and anti-atherogenic properties[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Tormentic acid inhibits LPS-induced iNOS, COX-2, and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages[1]. Tormentic acid suppresses high-fat diet-induced diabetes and hyperlipidemia by glucose transporter 4 and AMP-activated protein kinase phosphorylation[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 602.7±55.0 °C at 760 mmHg |
| Molecular Formula | C30H48O5 |
| Molecular Weight | 488.699 |
| Flash Point | 332.3±28.0 °C |
| Exact Mass | 488.350189 |
| PSA | 97.99000 |
| LogP | 6.21 |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.580 |
| Hazard Codes | Xi |
|---|