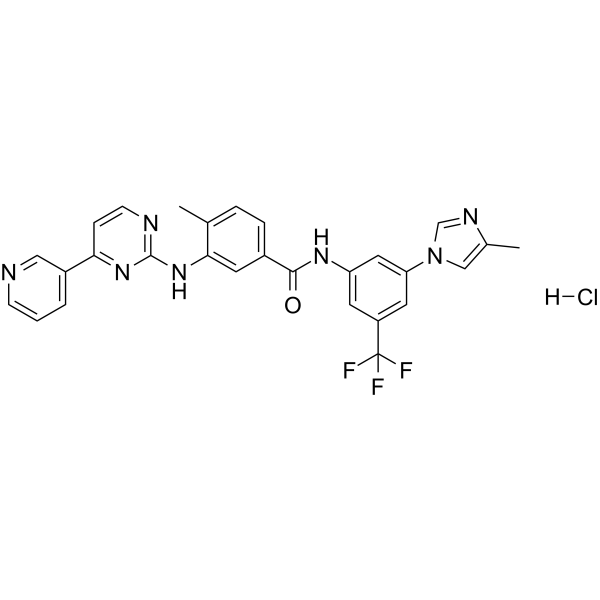
923288-95-3
| Name | 4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide,hydrochloride |
|---|---|
| Synonyms |
Benzamide, 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-, hydrochloride (1:1)
4-Methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluormethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzolcarboxamidhydrochlorid 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide hydrochloride Nilotinib hydrochloride anhydrous X8407 4-méthyl-N-[3-(4-méthyl-1H-imidazol-1-yl)-5-(trifluorométhyl)phényl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide chlorhydrate benzamide, 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-, monohydrochloride Nilotinib hydrochloride 4-Methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-{[4-(3-pyridinyl)-2-pyrimidinyl]amino}benzamide hydrochloride (1:1) UNII-K37N7BYX3X K37N7BYX3X Nilotinib HCl |
| Description | Nilotinib (AMN107) hydrochlorid is an orally available Bcr-Abl tyrosine kinase inhibitor with antineoplastic activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Nilotinib hydrochlorid, selective Abl inhibitor, is designed to interact with the ATP-binding site of BCR-ABL with a higher affinity than imatinib while being significantly more potent compared with imatinib (IC50<30 nM), also maintains activity against most of the BCR-ABL point mutants that confer Imatinib resistance[1]. Nilotinib hydrochlorid demonstrates significant antitumor efficacy against GIST xenograft lines and imatinib-resistant GIST cell lines which parent cell lines GK1C and GK3C shows imatinib sensitivity with IC50 of 4.59±0.97 µM and 11.15±1.48 µM, respectively, imatinib-resistant cell lines GK1C-IR and GK3C-IR shows Imatinib resistance with IC50 values of 11.74±0.17 µM (P<0.001) and 41.37±1.07 µM (P<0.001), respectively[2]. |
| In Vivo | Nilotinib hydrochlorid (oral gavage, 40 mg/kg, daily, 4 weeks) shows equivalent or higher antitumor effects in BALB/cSLc-nu/nu mice with GIST xenograft[2]. Nilotinib hydrochlorid has a significant healing effect on the macroscopic and microscopic pathologic scores and ensures considerable mucosal healing in the indomethacin-induced enterocolitis rat model while decreases the PDGFR α and β levels and apoptotic scores in the colon[3]. Animal Model: BALB/cSLc-nu/nu mice with GIST xenograft (GK1X, GK2X and GK3X)[2] Dosage: 40 mg/kg Administration: Oral gavage; daily; 4 weeks Result: Inhibited tumor growth by 69.6% in GK1X, 85.3% in GK2X and 47.5% in GK3X xenograft line. |
| References |
| Molecular Formula | C28H23ClF3N7O |
|---|---|
| Molecular Weight | 565.977 |
| Exact Mass | 565.160461 |
| PSA | 101.11000 |
| LogP | 7.61480 |
| Storage condition | -20℃ |