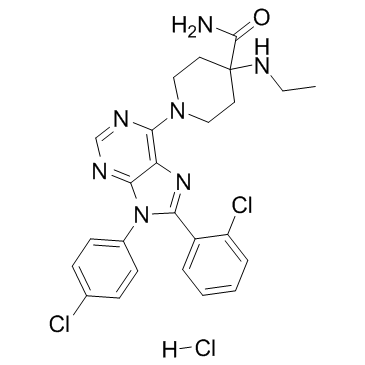
686347-12-6
| Name | 4-Piperidinecarboxamide, 1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-6-yl]-4-(ethylamino)-, hydrochloride (1:1) |
|---|---|
| Synonyms |
4-Piperidinecarboxamide, 1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-6-yl]-4-(ethylamino)-, hydrochloride (1:1)
1-[8-(2-Chlorophenyl)-9-(4-chlorophenyl)-9H-purin-6-yl]-4-(ethylamino)-4-piperidinecarboxamide hydrochloride (1:1) Otenabant HCl Otenabant hydrochloride CP945598 Otenabant (Hydrochloride) |
| Description | Otenabant Hydrochloride is a potent and selective cannabinoid receptor CB1 antagonist with Ki of 0.7 nM, exhibits 10,000-fold greater selectivity against human CB2 receptor. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.7 nM (CB1) |
| In Vitro | Otenabant HCl has low affinity with Ki of 7.6 μM for human CB2 receptors[1]. Otenabant HCl inhibits CB1 receptor with moderate unbound microsomal clearance, low hERG affinity, and adequate CNS penetration[2]. |
| In Vivo | Otenabant acutely stimulates energy expenditure in rats and decreases the respiratory quotient indicating a metabolic switch to increased fat oxidation. Otenabant (10 mg/kg, p.o.) promotes a 9%, vehicle adjusted weight loss in a 10 day weight loss study in diet-induced obese mice[1]. Otenabant HCl reverses four cannabinoid agonistmediated behaviors (locomotor activity, hypothermia, analgesia, and catalepsy) following administration of the synthetic CB1 receptor agonist CP-55940. Otenabant HCl exhibits dose-dependent anorectic activity in a model of acute food intake in rodents and increased energy expenditure and fat oxidation[2]. |
| Kinase Assay | Membranes are prepared from CHOK1 cells stably transfected with the human CB-1 receptor cDNA. GTPγ [35S] binding assays are performed in a 96-well FlashPlate format in duplicate using 100 pM GTPγ [35S] and 10μg membrane per well in assay buffer composed of 50 mM Tris HCl, pH 7.4, 3 mM MgCl2, pH 7.4, 10 mM MgCl2, 20 mM EGTA, 100 mM NaCl, 30 µM GDP, 0.1% bovine serum albumin, and the following protease inhibitors: 100 μg/mL bacitracin, 100 μg/mL benzamidine, 5 μg/mL aprotinin, 5 μg/mL leupeptin. The assay mix is then incubated with increasing concentrations of antagonist (10-10M to 10-5 M) for 10 min and challenged with the cannabinoid agonist CP-55,940 (10 μM). Assays are performed at 30°C for 1 h. The FlashPlates are then centrifuged at 2000 g for 10 min. Stimulation of GTPγ [35S] binding is then quantified using a Wallac Microbeta. EC50 calculations are done using Prism by GraphPad. Inverse agonism is measured in the absence of agonist. |
| Animal Admin | Male, 14 week old C57/Bl6/6J mice which has been maintained on a high fat diet (45% kcal from fat) for 6 weeks are selected for the DIO weight loss study. The animals body weights range at least five standard deviations from age-matched chow-fed control animals mean body weight. Mice are singly housed. The mean starting weight of all animals is 38.9±0.5 g. On day 0, mice are randomLy assigned to treatment groups (n=10 per group). Mice are dosed daily with vehicle or 10 mg/kg (p.o.) CP-945,598 over 10 days, starting approximately at 30 min before the start of the 12 h dark cycle. BW and food intake are recorded daily. Analysis of variance and comparison of means are calculated for daily and cumulative FI and cumulative BW measurements. P < 0.05 is considered statistically significant. |
| References |
| Melting Point | 275-276℃ (decomposition) |
|---|---|
| Molecular Formula | C25H26Cl3N7O |
| Molecular Weight | 546.879 |
| Exact Mass | 545.126465 |
| PSA | 101.96000 |
| LogP | 6.18130 |
| Appearance | white to beige |
| Storage condition | room temp |
| Water Solubility | DMSO: soluble1mg/mL, clear (warmed) |
| RIDADR | NONH for all modes of transport |
|---|