101246-66-6
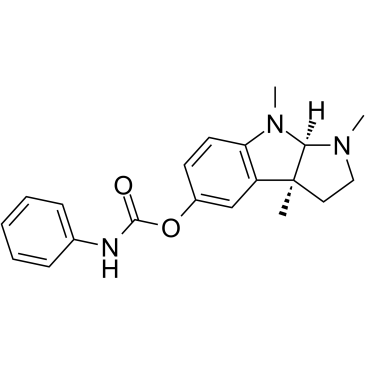
| Name | Phenserine,(3aS,8aR)-1,2,3,3a,8,8a-Hexahydro-1,3a,8-trimethylpyrrolo[2,3-b]indol-5-ol5-(N-phenylcarbamate) |
|---|
| Description | Phenserine ((-)-Eseroline phenylcarbamate) is a derivative of Physostigmine and is a potent, noncompetitive, long-acting and selective AChE inhibitor. Phenserine reduces β-amyloid precursor protein (APP) and β-amyloid peptide (Aβ) formation. Phenserine improves cognitive performance and attenuates the progression of Alzheimer's disease[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
AChE; β-amyloid precursor protein; β-amyloid peptide[1] |
| In Vitro | Phenserine (1-25 μM; 48 hours; CHO APP751SW cells) treatment CHO APP751SW cell shows 18.6% reduction in cells treated with 10 μM of Phenserine, while 25 μM concentration of Phenserine reduces APP level by 51.4%[2]. Western Blot Analysis[2] Cell Line: CHO APP751SW cells Concentration: 1 μM, 2.5 μM, 5 μM, 10 μM, 25 μM Incubation Time: 48 hours Result: 8.6% reduction in cells treated with 10 μM, while 25 μM concentration reduces APP level by 51.4%. |
| In Vivo | Phenserine (1-4 mg/kg; intraperitoneal injection; for 4 days; male Fischer-344 rats) treatment improves learning when cholinergic function has been impaired in a spatial memory task[3]. Animal Model: Male Fischer-344 rats (5 months old) induced by scopolamine[3] Dosage: 1 mg/kg, 2 mg/kg, 4 mg/kg Administration: Intraperitoneal injection; for 4 days Result: Improved morris water maze performance of scopolamine-treated rats.. |
| References |
[1]. Klein J. Phenserine. Expert Opin Investig Drugs. 2007 Jul;16(7):1087-97. |
| Density | 1.228g/cm3 |
|---|---|
| Boiling Point | 468.7ºC at 760mmHg |
| Molecular Formula | C20H23N3O2 |
| Molecular Weight | 337.41600 |
| Flash Point | 237.3ºC |
| Exact Mass | 337.17900 |
| PSA | 44.81000 |
| LogP | 3.74250 |
| Vapour Pressure | 5.85E-09mmHg at 25°C |
| Index of Refraction | 1.633 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| RTECS | UY8586000 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |