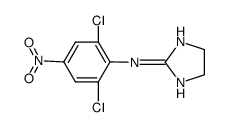
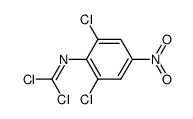
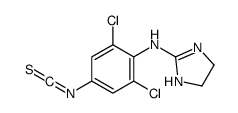
66711-21-5
| Name | apraclonidine |
|---|---|
| Synonyms |
para-aminoclonidine
4-Aminoclonidine Apraclonidinum Iopidine Apraclonidina Apraclonidinum [INN-Latin] 2,6-dichloro-1-N-(4,5-dihydro-1H-imidazol-2-yl)benzene-1,4-diamine 2-(4-amino-2,6-dichlorophenylamino)-2-imidazoline [3H]-p-aminoclonidine 2,6-Dichloro-N1-(4,5-dihydro-1H-imidazol-2-yl)benzene-1,4-diamine p-amino clonidine Apraclonidina [INN-Spanish] MFCD00865638 Aplonidine |
| Description | Apraclonidine hydrochloride (ALO 2145), a selective α2 and weak α1 receptor agonist activity, effectively lowers intraocular pressure (IOP) in human eyes. Apraclonidine hydrochloride is a topical ophthalmic solution[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Apraclonidine hydrochloride (ALO 2145) is more commonly used topically for glaucoma, as it penetrates the cornea and blood-brain barrier to a lesser extent and, thus, has fewer adverse systemic effects[2]. |
| In Vivo | Apraclonidine hydrochloride (ALO 2145) is effective in animal models of elevated IOP as well as glaucoma in humans. The ocular hypotensive effects of Apraclonidine are usually attributed to reduced aqueous humor synthesis and vasoconstrictor actions at the anterior segment branches of the ophthalmic artery. Apraclonidine (1.15%, single instillation) inhibits 98% of PGE2-induced aqueous flare elevationy[2][3] . Animal Model: male rabbits[3] Dosage: 1.15% Administration: Apraclonidine (1.15%, single instillation) Result: Inhibited PGE2-induced elevation of aqueous flare in pigmented rabbits. |
| References |
[1]. Subhashie Wijemanne, et al. Apraclonidine in the treatment of ptosis. J Neurol Sci |
| Density | 1.63g/cm3 |
|---|---|
| Boiling Point | 395.5ºC at 760mmHg |
| Molecular Formula | C9H10Cl2N4 |
| Molecular Weight | 245.10800 |
| Flash Point | 193ºC |
| Exact Mass | 244.02800 |
| PSA | 62.44000 |
| LogP | 2.36530 |
| Appearance | solid | white |
| Storage condition | 2-8°C |
| Water Solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1.4 mg/mL Solutions may be stored for several days at 4?#x00b0;C |
| Hazard Codes | T |
|---|---|
| Risk Phrases | R23/24/25 |
| Safety Phrases | S22-S36/37/39-S45 |
| RIDADR | UN 2811 6.1/PG 1 |
| WGK Germany | 3 |
| HS Code | 2933290090 |
|
~79% 
66711-21-5 |
| Literature: Van Dort; Neubig; Counsell Journal of medicinal chemistry, 1987 , vol. 30, # 7 p. 1241 - 1244 |
|
~% 
66711-21-5 |
| Literature: Journal of medicinal chemistry, , vol. 30, # 7 p. 1241 - 1244 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |