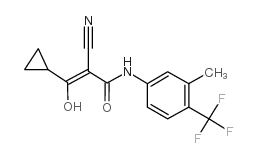
147076-36-6
| Name | (Z)-2-cyano-3-cyclopropyl-3-hydroxy-N-[3-methyl-4-(trifluoromethyl)phenyl]prop-2-enamide |
|---|---|
| Synonyms | Laflunimus |
| Description | Laflunimus (HR325) is an immunosuppressive agent and an analogue of the Leflunomide-active metabolite A77 1726. Laflunimus is an orally active inhibitor of dihydroorotate dehydrogenase (DHODH). Laflunimus suppresses immunoglobulin (Ig) secretion, with IC50 values of 2.5 and 2 µM for IgM and IgG, respectively. Laflunimus also is a prostaglandin endoperoxide H synthase (PGHS) -1 and -2 inhibitor[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Ig secretion from mouse splenocytes was induced by lipopolysaccharide (LPS) for 5 days. Laflunimus inhibited the secretion of IgM and IgG with IC50 values of 2.5 and 2 µM , respectively. Adding Uridine (50 µ M) increased these values to 70 and 60 µ M, respectively. Laflunimus inhibits LPS-induced kappa light-chain cell surface expression on 70Z/3 cells, a property also reversed by uridine[1]. Laflunimus (HR325) is more potent than A77 1726 as an inhibitor of PGHS in guinea pig polymorphonuclear leukocytes (IC50= 415 and 4400 nM, respectively) and on isolated ovine PGHS-1 (IC50=64 and 742 µM) and PGHS-2 (IC50=100 and 2766 µM)[2]. |
| In Vivo | HR325 (50 mg/kg; p.o.; days 14-18 after being injected with SRBC) inhibits the secondary anti-sheep red blood cell (SRBC) antibody response with ID50 values of 38 mg/kg[1]. Animal Model: Male CD-1 mice (20-24 g)[1] Dosage: 50 mg/kg Administration: Oral; days 14-18 after being injected with SRBC Result: The level of circulating anti-SRBC IgG in this model was inhibited dose relatedly by orally administered HR325. |
| References |
| Density | 1.441g/cm3 |
|---|---|
| Boiling Point | 408.7ºC at 760mmHg |
| Molecular Formula | C15H13F3N2O2 |
| Molecular Weight | 310.27100 |
| Flash Point | 201ºC |
| Exact Mass | 310.09300 |
| PSA | 73.12000 |
| LogP | 3.77098 |
| Vapour Pressure | 2.06E-07mmHg at 25°C |
| Index of Refraction | 1.588 |
| Hazard Codes | Xi |
|---|