476436-68-7
| Name | Naveglitazar |
|---|---|
| Synonyms |
LY 519818
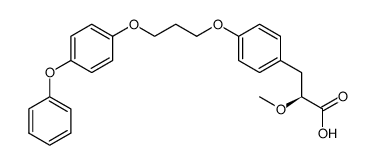
navaglitazar (2S)-2-methoxy-3-{4-[3-(4-phenoxyphenoxy)propoxy]phenyl}propanoic acid 2(S)-methoxy-3-{4-[3-(4-phenoxyphenoxy)propoxy]phenyl}propionic acid (2S)-2-methoxy-3-{4-[3-(4-phenoxyphenoxy)propoxy] phenyl}propanoic naveglitazar 2-methoxy-3-[4-[3-(4-phenoxyphenoxy)propoxy]phenyl]propanoic acid |
| Description | Naveglitazar (LY519818) is a nonthiozolidinedione peroxisome proliferator-activated receptor (PPAR) α-γ dual, γ-dominant agonist that has shown glucose-lowering potential in animal models[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Naveglitazar, a non-thiazolidinediones (TZD), functions as a potent and efficacious insulin sensitizer in rodents, possessing a novel profile that may result in an improved therapeutic agent for the treatment of type 2 diabetes and associated dyslipidemia. The extent of in vitro binding of [3H]Naveglitazar to plasma proteins is evaluated by ultracentrifugation in mouse, rat, and monkey plasma. The mean percentages±S.E.M. of protein binding of radioactivity in plasma over the concentration range of 0.1 to 1000 ng/ml after in vitro incubation at 37°C for 60 min are 99.5%±0.1% (mice), 99.6%±0.1% (rat), and 99.6%±0.3% (monkey). These results show that Naveglitazar is highly bound to plasma proteins among the species examined, and binding is independent of concentration[1]. |
| In Vivo | [14C]Naveglitazar is quickly absorbed and moderately metabolized before elimination. After oral administration, 47, 31, and 62% of the radioactivity, as assessed by AUC values, are circulating as metabolites in mice, rats, and monkeys, respectively. Half-lives for Naveglitazar and radioactivity are similar within each species; however, monkeys have substantially longer half-lives in comparison to mice and rats. Naveglitazar and radioactivity are slowly cleared from the system circulation in all species evaluated[1]. |
| Animal Admin | The single-dose plasma pharmacokinetics of Naveglitazar, LY591026, and radioactivity are studied in male ICR mice, and male Fischer 344 rats after oral administration of [14C]Naveglitazar. A separate study is conducted in rats to evaluate the pharmacokinetics of Naveglitazar and LY591026 after the administration of a single 10 mg/kg dose of each compound. Mice and rats are administered a 10 mg/kg oral dose of [14C]Naveglitazar with a specific activity of 50 μCi/kg[1]. Mice[1] Mice are divided into three groups. Group 1 animals (n=3 per time point) are housed in shoebox cages and are used to determine the pharmacokinetics of Naveglitazar, LY591026, and radioactivity. Blood samples are collected for plasma from mice via cardiac puncture at 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 120 h postdose. Group 2 mice are housed in metabolism cages (n=3 per cage) for the collection of excreta to determine the mass balance and metabolism of Naveglitazar. Urine, feces, and cage wash are collected in 24 h intervals for up to 120 h. The third group of animals (n=3 per time point) are housed in shoebox cages and used to evaluate the metabolites present in plasma. Rat[1] Rats are divided into three groups. Group 1 animals (n=3 per time point) are housed in shoebox cages and are used to determine the pharmacokinetics of Naveglitazar, LY591026, and radioactivity. Blood samples are collected for plasma from rats via cardiac puncture at 0.5, 1, 2, 4, 8, 12, 24, 48, and 72 h postdose. Group 2 rats (n=4) are individually housed in metabolism cages for the collection of excreta to determine the mass balance and metabolism of Naveglitazar. Urine, feces, and cage wash are collected in 24 h intervals for up to 120 h. Group 3 animals, which are bile-cannulated rats (n=4), are individually housed in metabolism cages for the collection of excreta to determine the mass balance and metabolism of Naveglitazar. Urine, feces, and cage wash are collected in 24 h intervals for up to 72 h, whereas bile is collected in 8-h intervals. In a separate study, rats are given Naveglitazar or LY591026. In this study, blood samples for plasma are collected at 0.5, 1, 2, 4, 8, 12, and 24 h to determine pharmacokinetics of each analyte. |
| References |
| Molecular Formula | C25H26O6 |
|---|---|
| Molecular Weight | 422.47000 |
| Exact Mass | 422.17300 |
| PSA | 74.22000 |
| LogP | 4.96890 |