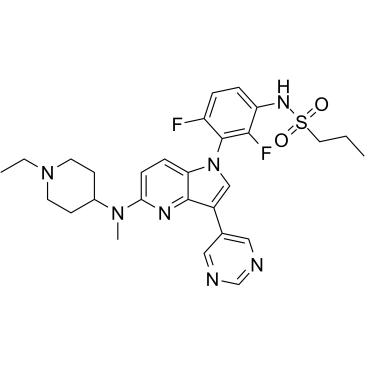
1392429-79-6
| Name | N-{3-[5-{[(1-Ethyl-4-piperidinyl)methyl]amino}-3-(5-pyrimidinyl)-1H-pyrrolo[3,2-b]pyridin-1-yl]-2,4-difluorophenyl}-1-propanesulfonamide |
|---|---|
| Synonyms |
1-Propanesulfonamide, N-[3-[5-[[(1-ethyl-4-piperidinyl)methyl]amino]-3-(5-pyrimidinyl)-1H-pyrrolo[3,2-b]pyridin-1-yl]-2,4-difluorophenyl]-
N-{3-[5-{[(1-Ethyl-4-piperidinyl)methyl]amino}-3-(5-pyrimidinyl)-1H-pyrrolo[3,2-b]pyridin-1-yl]-2,4-difluorophenyl}-1-propanesulfonamide |
| Description | BI-882370 is a potent and selective RAF kinase inhibitor that binds to the ATP binding site of the kinase positioned in the DFG-out (inactive) conformation of the BRAF kinase. BI-882370 (BI 882370) inhibits the oncogenic BRAFV600E-mutant, the WT BRAF and CRAF kinases with IC50s of 0.4, 0.8, and 0.6 nM, respectively. BI-882370 also inhibits SRC family kinases[1]. |
|---|---|
| Related Catalog | |
| Target |
Braf:0.6 nM (IC50) c-Raf:0.8 nM (IC50) BRafV600E:0.4 nM (IC50) |
| In Vitro | BI-882370 (0.9-6000 nM; 3 days) inhibits the BRAF-mutant human melanoma and colorectal cancer cells proliferation with a EC50 range of 1-10 nM[1]. BI-882370 (0.1-100 nM, 0.1-3000 nM; 2 hours, 24 hours) results in a reduction of p-MEK1/2, p-ERK1/2 and cyclin D1/D2 expression in BRAFV600E-mutant A375 cells; induces p-MEK1/2 and enhances p-ERK1/2 , cyclins D1/D2 or Kip1/p27 is not affected in BRO cells[1]. Cell Proliferation Assay[1] Cell Line: BRAF-mutant and WT melanoma cell lines (A101D, A375, SK-MEL-28, G-361, and BRO); Colorectal cancer cell lines (COLO 205, HT-29, LS411N, and HCT-116) Concentration: 0.9-6000 nM Incubation Time: 3 days Result: Showed a EC50 range of 1-10 nM in an extended panel of BRAF-mutant human melanoma and colorectal cancer cell; while proliferation of BRAF WT cells was inhibited with EC50 >1μM. Western Blot Analysis[1] Cell Line: BRAFV600E-mutant A375 cells; BRAF WT, NRAS-mutant BRO (WT BRO) cells Concentration: 0.1-100 nM; 0.1-3000 nM Incubation Time: 2 hours; 24 hours Result: Resulted in a reduction of phospho-MEK1/2 signals. |
| In Vivo | BI-882370 (deliver orally; 25 mg/kg, 50 mg/kg; twice daily; 2 weeks) is efficacious in multiple mouse models of BRAF-mutant melanomas and colorectal carcinomas, shows superior efficacy compared with Vemurafenib, Dabrafenib, or Trametinib[1]. BI-882370 (deliver orally; 25 mg/kg; twice daily; 40 days) developes resistance within 3 weeks, but resistance is not observed during 5 weeks of second-line therapy in combination with trametinib[1]. BI-882370 (deliver orally; 60 mg/kg; once daily; 2 weeks) indicates lack of toxicity in terms of clinical chemistry, hematology, pathology, and toxicogenomics in rats[1]. Animal Model: Human melanoma xenografts in nude mice with BRAF-mutant melanomas and colorectal carcinomas cells (A375, COLO 205; G-361, HT-29 cells)[1] Dosage: 25 mg/kg; 50 mg/kg Administration: Deliver orally; 25 mg/kg, 50 mg/kg; twice daily; 2 weeks Result: Regressed tumors partially, upon discontinuation, tumor regrowth was markedly delayed. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 666.8±65.0 °C at 760 mmHg |
| Molecular Formula | C28H33F2N7O2S |
| Molecular Weight | 569.669 |
| Flash Point | 357.0±34.3 °C |
| Exact Mass | 569.238464 |
| LogP | 2.59 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.660 |