CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UV9100000
-
CHEMICAL NAME :
-
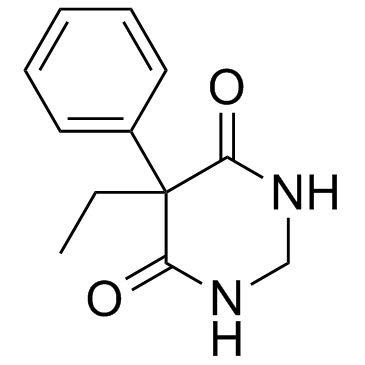
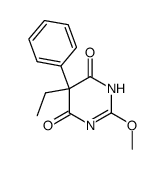
4,6(1H,5H)-Pyrimidinedione, 5-ethyldihydro-5-phenyl-
-
CAS REGISTRY NUMBER :
-
125-33-7
-
BEILSTEIN REFERENCE NO. :
-
0218034
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
33
-
MOLECULAR FORMULA :
-
C12-H14-N2-O2
-
MOLECULAR WEIGHT :
-
218.28
-
WISWESSER LINE NOTATION :
-
T6MV DVMTJ C2 CR
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
38 gm/kg/7Y-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - toxic psychosis
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
240 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
280 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
332 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - anticonvulsant
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
340 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - anticonvulsant
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
10500 mg/kg/14D-C
-
TOXIC EFFECTS :
-
Behavioral - ataxia Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
17062 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Liver - other changes Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
27440 mg/kg/14D-C
-
TOXIC EFFECTS :
-
Behavioral - ataxia Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
89 gm/kg/13W-C
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - ataxia Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
2 gm/kg/4D-I
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - ataxia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10350 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - skin and skin appendages Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10350 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other neonatal measures or effects Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2025 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - Apgar score (human only)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
10640 mg/kg
-
SEX/DURATION :
-
female 1-38 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - drug dependence Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
330 mg/kg
-
SEX/DURATION :
-
female 6-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
990 mg/kg
-
SEX/DURATION :
-
female 6-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
875 mg/kg
-
SEX/DURATION :
-
male 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
275 mg/kg
-
SEX/DURATION :
-
female 6-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
Sperm Morphology
MUTATION DATA
-
TYPE OF TEST :
-
Micronucleus test
-
TEST SYSTEM :
-
Rodent - hamster Embryo
-
DOSE/DURATION :
-
250 mg/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 392,61,1997 *** REVIEWS *** TOXICOLOGY REVIEW DICPBB Drug Intelligence and Clinical Pharmacy. (POB 42435, Cincinnati, OH 45242) V.3- 1969- Volume(issue)/page/year: 8,690,1974 TOXICOLOGY REVIEW AUHPAI Australian Journal of Hospital Pharmacy. (B.R. Miller, POB 125, Heidelberg, Vic., Australia) V.1- 1971- Volume(issue)/page/year: 4(1),5,1974 TOXICOLOGY REVIEW DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. (ADIS Press International Inc., Suite B-30, Oxford Ct. Business Center, 582 Middletown Blvd., Langhorne, PA 19047) V.1- 1971- Volume(issue)/page/year: 8,354,1974 TOXICOLOGY REVIEW PRSMA4 Proceedings of the Royal Society of Medicine. (New York, NY) V.1-70, 1907-77. For publisher information, see JRSMD9. Volume(issue)/page/year: 63,48,1970 TOXICOLOGY RIVIEW BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 2,442,1973 TOXICOLOGY REVIEW AJDCAI American Journal of Diseases of Children. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1-80(3), 1911-50; V.100- 1960- Volume(issue)/page/year: 131,1337,1977 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4105 No. of Facilities: 416 (estimated) No. of Industries: 3 No. of Occupations: 6 No. of Employees: 11085 (estimated) No. of Female Employees: 6869 (estimated)
|