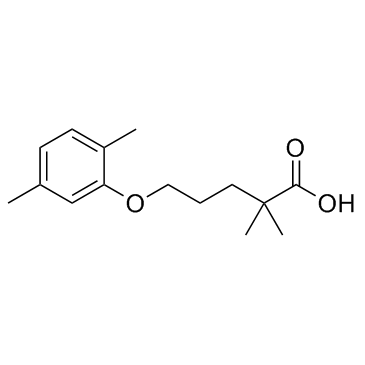
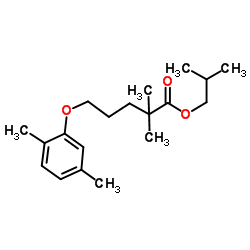
25812-30-0
| Name | gemfibrozil |
|---|---|
| Synonyms |
Pentanoic acid, 5-(2,5-dimethylphenoxy)-2,2-dimethyl-
GEMFIBROZIL,USP 2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeric Acid Genlip 5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic acid EINECS 247-280-2 lopid CI-7 2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid (2,2-Dimethyl-5-(2,5-xylyloxy)valeric acid gevilon Lipttr ci-719 2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic Acid Decrelip Gemfibrozil MFCD00079335 C1-719 Lipozid |
| Description | Gemfibrozil is an activator of PPAR-α, used as a lipid-lowering drug; Gemfibrozil is also a nonselective inhibitor of several P450 isoforms, with Ki values for CYP2C9, 2C19, 2C8, and 1A2 of 5.8, 24, 69, and 82 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
PPAR-α CYP2C9:5.8 μM (Ki) CYP2C19:24 μM (Ki) CYP2C8:69 μM (Ki) CYP1A2:82 μM (Ki) |
| In Vitro | Gemfibrozil is an activator of PPAR-α, used as a lipid-lowering drug[1]; also a nonselective inhibitor of several P450 isoforms, with Ki values for CYP2C9, 2C19, 2C8, and 1A2 of 5.8, 24, 69, and 82 μM, respectively[3]. Gemfibrozil (100, 150, 200 μM) inhibits the cytokine-induced NO production in a concentration dependent manner in human U373MG astroglial cells, and such effects are not due to any change of the stability of iNOS mRNA. Gemfibrozil (50, 100, 200 μM) inhibits human iNOS promoter-derived luciferase activity in cytokine-stimulated human U373MG astroglial cells. Furthermore, Gemfibrozil (50, 100, 150, and 200 μM) shows no effects on the viability of the cells[1]. Gemfibrozil considerably inhibits both M-23 and M-1 formation (catalyzed by CYP2C8 and CYP3A4), with Ki (IC50) values of 69 μM (95 μM) and 273 μM (>250 μM), respectively, in human liver microsomes. Gemfibrozil (0-250 μM) dose dependently inhibits the formation of M-23 (IC50, 68 μM) and M-1 (IC50, 78 μM) in recombinant CYP2C8, but shows no appreciable effect on the formation of these metabolites in recombinant CYP3A4[3]. |
| In Vivo | Gemfibrozil (62 mg/kg/day, p.o.) treatment initiated 3 days before spinal cord injury (SCI) causes decreased locomotor function, and induces a trend for decreased white matter sparing after injury in mice. Gemfibrozil (62 mg/kg/day, p.o.) decreases macrophage immunoreactivity but increases T cell infiltration into spared tissue[2]. |
| Kinase Assay | Activities of CYP3A4 (testosterone 6β-hydroxylation) and CYP2C8 (paclitaxel 6α-hydroxylation) are determined. The marker substrate concentrations used, 25 μM testosterone and 1 to 5 μM paclitaxel, are comparable with or around the Km values of the reactions. Gemfibrozil is either coincubated at 37°C for 15 min with the marker substrate and NADPH (1 mM) before the reaction is initiated with human liver microsomes (0.1 mg/mL) or preincubated with human liver microsomes and NADPH for 15 min before adding the marker substrate[3]. |
| Cell Assay | Briefly, 400 μL of culture supernatant is allowed to react with 200 μL of Griess reagent and incubated at room temperature for 15 min. The optical density of the assay samples is measured spectrophotometrically at 570 nm. Fresh culture medium serves as the blank in all experiments. Nitrite concentrations are calculated from a standard curve derived from the reaction of NaNO2 in the assay[1]. |
| Animal Admin | Mice are given vehicle or gemfibrozil beginning 3 days before SCI to ensure drug availability at the time of the injury (n=5-6/group). The drug is delivered orally by dissolving it in ethanol (0.25% w/w of gemfibrozil) and coating food pellets such that each animal receives appr 62 mg/kg/day; chow for control groups is treated with ethanol. Ethanol for each group is allowed to completely evaporate before giving the food to the animals. In addition, animals receive an intraperitoneal injection of vehicle or gemfibrozil (500 µg dissolved in 200 µL PBS) 1 h after the injury, and then continued to receive the drug in their food until the end of the study (28 days post-injury)[2]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 394.7±30.0 °C at 760 mmHg |
| Melting Point | 61-63°C |
| Molecular Formula | C15H22O3 |
| Molecular Weight | 250.333 |
| Flash Point | 141.6±18.1 °C |
| Exact Mass | 250.156891 |
| PSA | 46.53000 |
| LogP | 4.39 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.512 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YV7120000 |
| HS Code | 2918990090 |
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |