82854-37-3
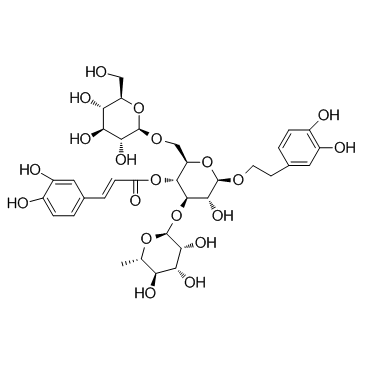
| Name | [(2R,3R,4R,5R,6R)-6-[2-(3,4-dihydroxyphenyl)ethoxy]-5-hydroxy-2-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate |
|---|---|
| Synonyms |
(2R,3R,4R,5R,6R)-6-[2-(3,4-Dihydroxyphenyl)ethoxy]-5-hydroxy-2-({[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}methyl)-4-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-3-yl (2E)-3-(3,4-dihydroxyphenyl)acrylate
2-(3,4-Dihydroxyphenyl)ethyl 6-deoxy-α-L-mannopyranosyl-(1->3)-[β-D-glucopyranosyl-(1->6)]-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-β-D-glucopyranoside 2-(3,4-dihydroxyphenyl)ethyl 6-deoxy-α-L-mannopyranosyl-(1-3)-[β-D-glucopyranosyl-(1-6)]-4-O-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]-β-D-glucopyranoside 2-(3,4-Dihydroxyphenyl)ethyl-6-deoxy-α-L-mannopyranosyl-(1->3)-[β-D-glucopyranosyl-(1->6)]-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]-β-D-glucopyranoside β-D-Glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl O-6-deoxy-α-L-mannopyranosyl-(1->3)-O-[β-D-glucopyranosyl-(1->6)]-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]- β-D-glucopyranoside, 2-(3,4-dihydroxyphenyl)ethyl O-6-deoxy-α-L-mannopyranosyl-(1->3)-O-[β-D-glucopyranosyl-(1->6)]-4-O-[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]- Echinacoside 2-(3,4-Dihydroxyphenyl)ethyl 6-deoxy-α-L-mannopyranosyl-(1->3)-[β-D-glucopyranosyl-(1->6)]-4-O-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]-β-D-glucopyranoside UNII-I04O1DT48T |
| Description | Echinacoside is a natural polyphenolic compound, has various kinds of pharmacological activities, such as antioxidative, anti-inflammatory, neuroprotective, hepatoprotective, nitric oxide radical-scavenging and vasodilative ones.IC50 value:Target:in vitro: Echinacoside(ECH) dose dependently inhibited HEWL aggregation, and this inhibition occurred in different fiber-forming stages. ECH could also scavenge the DPPH and OH free radicals in a concentration-dependent manner. ECH could increase viability of rat pheochromocytoma PC12 cells injured by Aβ and suppress the increase in intracellular reactive oxygen species (ROS) triggered by Aβ [1]. Transient treatment with echinacoside inhibits cytochrome c release and caspase-3 activation caused by ensuing rotenone exposure via activating Trk-extracellular signal-regulated kinase (ERK) pathway in neuronal cells [2]. ECH caused a significant increase in cell proliferation, ALP activity, COL I contents, OCN levels and an enhancement of mineralization in osteoblasts at the concentration range from 0.01 to 10nmol·L(-1) (p<0.05), suggesting that ECH has a stimulatory effect on osteoblastic bone formation or has potential activity against osteoporosis [4]. in vivo: In OVX rats, the increases of body weight, serum hydroxyproline (HOP) levels, and the decreases of uterus wet weight and BMD were significantly reversed by ECH treatment [3]. Echinacoside (60 mg/kg) was given intraperitoneally to mice at 1 h prior to GalN/LPS exposure. Pretreatment with echinacoside remarkably improved the survival rate of GalN/LPS-treated mice and attenuated acute hepatotoxicity, as demonstrated by decreased ALT levels and improved histological signs. Echinacoside shows both anti-apoptotic and anti-inflammatory properties, characterized by a substantial inhibition of hepatocyte apoptosis and a significant reduction in the inflammatory markers, including myeloperoxidase, extracellular nucleosomes, high-mobility group box 1, and inflammatory cytokines in the plasma of mice, which may be important mechanisms related to its protective effect [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 1062.7±65.0 °C at 760 mmHg |
| Molecular Formula | C35H46O20 |
| Molecular Weight | 786.728 |
| Flash Point | 327.9±27.8 °C |
| Exact Mass | 786.258240 |
| PSA | 324.44000 |
| LogP | 0.14 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.698 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|