891016-02-7
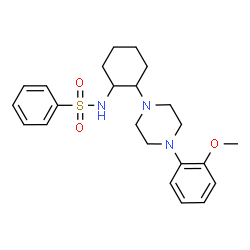
| Name | N-{2-[4-(2-Methoxyphenyl)-1-piperazinyl]cyclohexyl}benzenesulfonamide |
|---|---|
| Synonyms |
MFCD14811190
N-{2-[4-(2-Methoxyphenyl)-1-piperazinyl]cyclohexyl}benzenesulfonamide Benzenesulfonamide, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]cyclohexyl]- ML-SI3 |
| Description | ML-SI3 is a TRPML Channel Inhibitor. ML-SI3 blocks TRPML1 and TRPML2 with IC50s of 4.7 µM and 1.7 µM respectively. ML-SI3 prevents lysosomal calcium efflux and blocks downstream TRPML1-mediated induction of autophagy[1][5]. |
|---|---|
| Related Catalog | |
| Target |
TRPML1:4.7 μM (IC50) TRPML2:1.7 μM (IC50) TRPML3:12.5 μM (IC50) |
| In Vitro | ML-SI3 (10 μM) inhibits ML-SA1-evoked Ca2+ signals in HeLa cells[2]. ML-SI3 (25-75 μM, 24h) disrupts tegumental integrity of adult schistosomes[3]. ML-SI3 (10 μM) blocks Rapamycin (HY-10219)-evoked ITRPML1 in mimic of lysosomal lumen[4]. ML-SI3 (3 µM, 6 h) abolishes the increase in both LC3II and p62 levels induced by hypoxia/reoxygenation (H/R) (4 h H/2 h R) in neonatal rat ventricular myocytes (NRVM)[5]. |
| In Vivo | ML-SI3 (1.5 mg/kg, i.p., four times) attenuates I/R injury in mouse cardiomyocytes[5]. Animal Model: Myocardial Ischemia/reperfusion (I/R) injury mice[5] Dosage: 1.5 mg/kg Administration: Intraperitoneal injection (i.p.), four times before and during the in vivo I/R (ischemia 30 min and reperfusion 1 day) Result: Restored the blocked autophagic fux in the cardiomyocytes subjected to I/R. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 589.3±60.0 °C at 760 mmHg |
| Molecular Formula | C23H31N3O3S |
| Molecular Weight | 429.576 |
| Flash Point | 310.2±32.9 °C |
| Exact Mass | 429.208618 |
| LogP | 4.00 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.629 |
| Storage condition | -20°C |
| Hazard Codes | Xi |
|---|