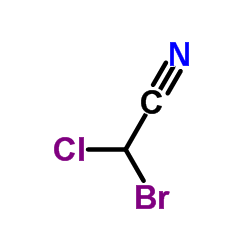
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
AL8010000
-
CHEMICAL NAME :
-
Acetonitrile, bromochloro-
-
CAS REGISTRY NUMBER :
-
83463-62-1
-
LAST UPDATED :
-
199607
-
DATA ITEMS CITED :
-
14
-
MOLECULAR FORMULA :
-
C2-H-Br-Cl-N
-
MOLECULAR WEIGHT :
-
154.40
-
WISWESSER LINE NOTATION :
-
NCYGE
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2400 mg/kg/2W-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
825 mg/kg
-
SEX/DURATION :
-
female 7-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
845 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
585 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
65 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TEST SYSTEM :
-
Rodent - hamster Ovary
-
DOSE/DURATION :
-
4200 nmol/L
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 5,1065,1985 *** REVIEWS *** IARC Cancer Review:Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 52,269,1991 IARC Cancer Review:Human No Available Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 52,269,1991 IARC Cancer Review:Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 52,269,1991
|