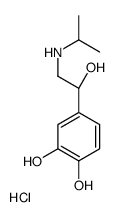
51-30-9
| Name | Isoprenaline hydrochloride |
|---|---|
| Synonyms |
Isoproterenol hydrochloride
Pyrocatechol, 4-(1-hydroxy-2-(isopropylamino)ethyl)-, hydrochloride Mistarel 4-(1-Hydroxy-2-(isopropylamino)ethyl)pyrocatechol hydrochloride 3,4-Dihydroxy-α-[(isopropylamino)methyl]benzyl alcohol hydrochloride 4-{1-hydroxy-2-[(1-methylethyl)amino]ethyl}benzene-1,2-diol hydrochloride Isovon euspiran Isoprenaline HCl 1,2-benzenediol, 4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-, hydrochloride 1,2-Benzenediol, 4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-, hydrochloride (1:1) EINECS 200-089-8 Saventrine IV (±)-Isoproterenol hydrochloride Suscardia 4-[1-hydroxy-2-(isopropylamino)ethyl]benzene-1,2-diol hydrochloride isoprenaline 4-[1-Hydroxy-2-(isopropylamino)ethyl]-1,2-benzenediol hydrochloride (1:1) Isomenyl 4-[1-Hydroxy-2-(isopropylamino)ethyl]benzene-1,2-diol hydrochloride (1:1) isuprel [3H]-Isoproterenol hydrochloride Isoprenaline (hydrochloride) DL-Isoproterenol hydrochloride N-Isopropyl-DL-noradrenaline hydrochloride Isoprenaline hydrochloride 4-[1-hydroxy-2-(propan-2-ylamino)ethyl]benzene-1,2-diol hydrochloride (1:1) MFCD00012603 Isoproterenol HCl proternol DL-Isoprenaline hydrochloride isadrine |
| Description | Isoprenaline hydrochloride is a non-selective beta-adrenergic receptor agonist with potent peripheral vasodilator, bronchodilator, and cardiac stimulating activities. |
|---|---|
| Related Catalog | |
| Target |
Beta-adrenergic receptor[1] |
| In Vitro | Isoprenaline (300 nM, 3 min) increases particulate cGMP- and cilostamide-inhibited, low-Km cAMP phosphodiesterase (cAMP-PDE) activity by about 100% in intact rat fat cells[1]. Isoprenaline inhibits insulin-stimulated glucose transport activity in rat adipocytes. Isoprenaline, in the absence of adenosine, promotes a time-dependent (t1/2 approximately 2 min) decrease in the accessibility of insulin-stimulated cell surface GLUT4 of > 50%, which directly correlated with the observed inhibition of transport activity[2]. Isoprenaline (5 nM and 10 mM) increases cyclic AMP levels and this effect is potentiated by cilostamide (10 mM), by rolipram, a cyclic AMP-specific PDE (PDE 4) inhibitor (10 mM) and by cyclic GMP-elevating agents (50 nM ANF or 30 nM SNP plus 100 nM DMPPO)[3]. Isoprenaline increases the transcriptional activity of Gi alpha-2 gene to 140% of the control value, whereas gene specific hybridization for Gs alpha remains unchanged[4]. Isoprenaline (20 nM) increases the amplitude of total iK and causes a negative shift of approximately 10 mV in the activation curve for iK, both in the absence and in the presence of 300 nM nisoldipine to block the L-type Ca2+ current. Isoprenaline (20 nM) increases the spontaneous pacemaker rate of sino-atrial node pacemaker cells by 16% in rabbit isolated pacemaker cells[5]. |
| Cell Assay | Cells are seeded in 24-well culture dishes at a density of 2 to 5×104 cells per well. Experiments are performed after 3 to 5 days in culture when cells has reached confluence. Culture medium is aspirated and replaced by 0.5 mL of PBS containing the pharmacological agents. Treatments are performed in quadruplicate at 37°C. The type 3, 4 and 5 PDE inhibitors cilostamide (10 gM), rolipram (10 pM) and DMPPO (10 gM), respectively, are incubated with cells for 30 min before addition of adenylate or guanylate cyclase activators. Cyclic GMP and cyclic AMP are respectively increased in RASMC by stimulation of particulate guanylate cyclase with ANF (50 nM for 10 min) or fl-adrenoceptors with isoprenaline (5 nm for 5 min). At the end of the incubation period, the medium is removed and intracellular cyclic nucleotides are extracted by two ethanolic (65%) ishes at 4°C for 5 min. Ethanolic extracts are pooled, evaporated to dryness by a Speed-Vac system. The dried extract is dissolved in a suitable amount of assay buffer and cyclic nucleotide levels are measured by scintillation proximity assay. |
| References |
| Density | 1.324 g/cm3 |
|---|---|
| Boiling Point | 417.5ºC at 760 mmHg |
| Melting Point | 165-175 °C (dec.)(lit.) |
| Molecular Formula | C11H18ClNO3 |
| Molecular Weight | 247.719 |
| Flash Point | 179.7ºC |
| Exact Mass | 247.097519 |
| PSA | 72.72000 |
| LogP | 2.32210 |
| Storage condition | Store at RT |
| Stability | Stable, but may be air and light sensitive. Incompatible with strong oxidizing agents. |
| Water Solubility | Soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DO1925000 |
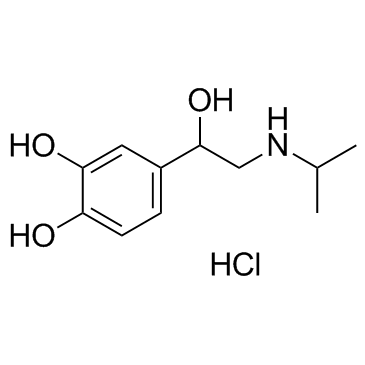
51-30-9 ~% 
51-30-9 |
| Literature: Venter, Daniel P. Tetrahedron, 1991 , vol. 47, # 27 p. 5019 - 5024 |
| Precursor 1 | |
|---|---|
| DownStream 3 | |