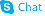56974-61-9
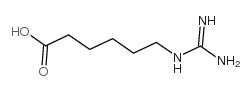
| Name | Gabexate Mesylate |
|---|---|
| Synonyms |
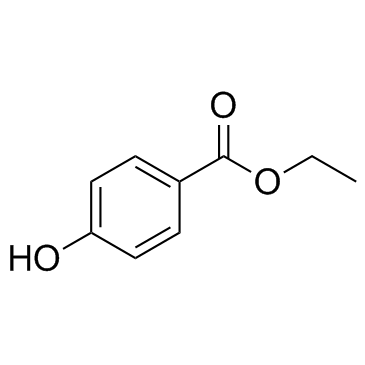
benzoic acid, 4-[[6-[(diaminomethylene)amino]-1-oxohexyl]oxy]-, ethyl ester, methanesulfonate (1:1)
ethyl 4-({6-[(diaminomethylidene)amino]hexanoyl}oxy)benzoate methanesulfonate (1:1) Ethyl 4-[(6-carbamimidamidohexanoyl)oxy]benzoate methanesulfonate (1:1) Gabexate monomethanesulfonate Benzoic acid, 4-[[6-[(aminoiminomethyl)amino]-1-oxohexyl]oxy]-, ethyl ester, methanesulfonate (1:1) MFCD00210299 Ethyl 4-({6-[(diaminomethylene)amino]hexanoyl}oxy)benzoate methanesulfonate (1:1) Gabexate mesylate |
| Description | Gabexate Mesylate is a Factor X inhibitor; serine protease inhibitor .Target: Factor XGabexate mesylate is a non-antigenic synthetic inhibitor of trypsin-like serine proteinases that is therapeutically used in the treatment of pancreatitis and disseminated intravascular coagulation and as a regional anticoagulant for hemodialysis. Values of the inhibition constant (K(i)) for gabexate mesylate binding to human and bovine tryptase were 3.4 x 10(-9) M and 1.8 x 10(-7) M (at pH 7.4 and 37.0 degrees ), respectively. Gabexate mesylate inhibited the fibrinogenolytic activity of human tryptase [1]. Gabexate Mesylate decreased the TNFalpha production of LPS-stimulated monocytes as shown by the inhibition of mRNA expression and increased the IL-10 production of LPS-stimulated monocytes. Gabexate Mesylate also suppressed the NFkappaB activity of LPS-stimulated monocytes. Inhibitory effect of Gabexate Mesylate on the TNFalpha production of activated human monocytes is mediated by the suppression of NFkappaB activation [2]. Gabexate mesylate inhibits competitively constitutive and inducible NO synthase (cNOS and iNOS, respectively), with Kivalues of 1.0×10?4M and 5.0×10?3M, respectively, at pH 7.4 and 37.0°C. gabexate mesylate increases iNOS mRNA expression in rat C6 glioma cells, as induced byE. colilipopolysaccharide plus interferon-γ. Gabexate mesylate inhibits dose-dependently nitrite production (i.e. NO release) in rat C6 glioma cells, as induced byE. colilipopolysaccharide plus interferon-γ [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 508.6ºC at 760 mmHg |
|---|---|
| Melting Point | 91 °C |
| Molecular Formula | C17H27N3O7S |
| Molecular Weight | 417.477 |
| Flash Point | 261.4ºC |
| Exact Mass | 417.156982 |
| PSA | 177.25000 |
| LogP | 3.58790 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DG2800800 |
|
~76% 
56974-61-9 |
| Literature: Isobe, Toshio; Ishikawa, Tsutomu Journal of Organic Chemistry, 1999 , vol. 64, # 19 p. 6984 - 6988 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |