55142-85-3
| Name | ticlopidine |
|---|---|
| Synonyms |
5-[(2-Chlorophenyl)methyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
TICLID 5-[(2-chlorophenyl)methyl]-6,7-dihydro-4H-thieno[3,2-c]pyridine MFCD00661081 Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro- 5-(o-Chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Ticlopidina 1,2,3,4-tetrahydro-N-<(2-chlorophenyl)methyl>-thieno<3,2-c>pyridine Ticlopidine Ticlopidine [INN:BAN] Ticlopidinum [INN-Latin] Ticlopidinum Thieno(3,2-c)pyridine, 5-((2-chlorophenyl)methyl)-4,5,6,7-tetrahydro- Ticlopidine (INN) 5-(2-Chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine UNII-OM90ZUW7M1 [14C]-Ticlopidine EINECS 259-498-5 Ticlopidina [INN-Spanish] |
| Description | Ticlopidine (PCR 5332), an antithrombotic prodrug, acts as an allosteric, noncompetitive inhibitor of CD39 with the IC50 of 81.7 µM. Ticlopidine blocks several NTPDase isoenzymes with IC50s of 170 µM and 149 µM for NTPDase2 and NTPDase3, respectively[1]. Ticlopidine is an inhibitor of CYP2C19 human liver cytochrome. Ticlopidine inhibits CYP2C9 and CYP3A4 with IC50s of 26.0 and 32.3 μM, respectively[2][3]. |
|---|---|
| Related Catalog | |
| Target |
Cytochrome P450[1] |
| In Vitro | Ticlopidine exhibits activity against human CD39 with apparent Ki,app values of 14 µM[1]. Ticlopidine inhibits recombinant human CD39 expressed in COS-7-cells with the Ki value of 127±12 µM[1]. Growth rate is affected during the first days of culture, Ticlopidine (30 and 150 µM) reducing its effect in the following days[4]. Cell Proliferation Assay[4] Cell Line: Human endothelial cells Concentration: 30 and 150 µM Incubation Time: 2, 6; 10 days Result: Treated cells grow slower if compared with controls and this effect correlates with the concentration of Ticlopidine in the culture medium. |
| In Vivo | Oral administration of Losartan with 10 mg/kg Ticlopidine significantly increases the AUC (by 65.0%), suggesting that Ticlopidine can effectively inhibit the metabolism of losartan in the intestine and/or liver[3]. Animal Model: Male Sprague-Dawley rats (7-8 weeks old, weighing 270-300 g)[3] Dosage: 4 or 10 mg/kg Administration: Orally administered 30 min before oral administration of losartan. Result: The AUC and Cmax of Losartan after oral administration with Losartan and 10 mg/kg Ticlopidine were significantly greater (by 65.0% and 49.4%, respectively) than those of control rats. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 367.3±37.0 °C at 760 mmHg |
| Molecular Formula | C14H14ClNS |
| Molecular Weight | 263.786 |
| Flash Point | 175.9±26.5 °C |
| Exact Mass | 263.053558 |
| PSA | 31.48000 |
| LogP | 3.77 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.638 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | C,Xi |
|---|---|
| Risk Phrases | R34:Causes burns. |
| Safety Phrases | S26-S27-S36/37/39-S45 |
| RIDADR | UN 3261 8/PG 3 |
| WGK Germany | 3 |
| HS Code | 2934999090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
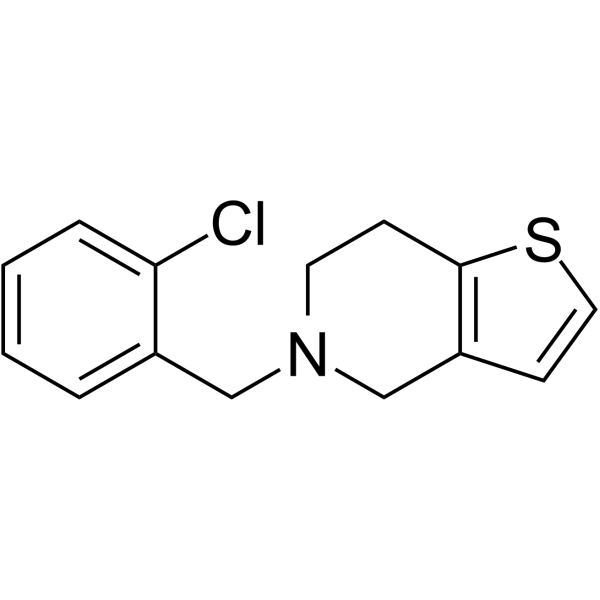

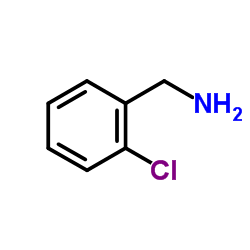
![4,5,6,7-Tetrahydrothieno[3,2-c]pyridine structure](https://image.chemsrc.com/caspic/421/54903-50-3.png)
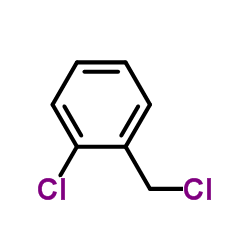

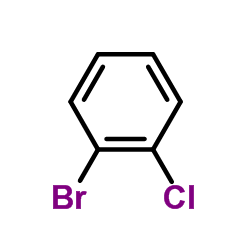
![5-(2-chloro-benzyl)-5,6-dihydro-4H-thieno[3,2-c]pyridin-7-one, hydrochloride structure](https://image.chemsrc.com/caspic/416/79714-07-1.png)

![5-[(2-Chlorophenyl)methyl]-5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one structure](https://image.chemsrc.com/caspic/418/83427-51-4.png)