CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XJ9089100
-
CHEMICAL NAME :
-
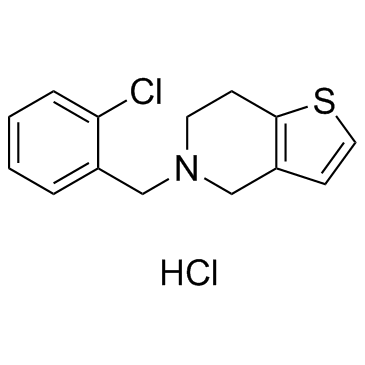
Thieno(3,2-c)pyridine, 4,5,6,7-tetrahydro-5-(o-chlorobenzyl)-, hydrochloride
-
CAS REGISTRY NUMBER :
-
53885-35-1
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
16
-
MOLECULAR FORMULA :
-
C14-H14-Cl-N-S.Cl-H
-
MOLECULAR WEIGHT :
-
300.26
-
WISWESSER LINE NOTATION :
-
T56 BS GN&TJ G1R BG &GH
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1780 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
MEPHDN Medical Pharmacy. (Daiichi Seiyaku K.K., 3-14-10 Nihonbashi, Chuo-ku, Tokyo 103, Japan) V.1- 1966- Volume(issue)/page/year: 15,272,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - other changes Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
YKYUA6 Yakkyoku. Pharmacy. (Nanzando, 4-1-11, Yushima, Bunkyo-ku, Tokyo, Japan) V.1- 1950- Volume(issue)/page/year: 32,1499,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
70 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 12,1204,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
MEPHDN Medical Pharmacy. (Daiichi Seiyaku K.K., 3-14-10 Nihonbashi, Chuo-ku, Tokyo 103, Japan) V.1- 1966- Volume(issue)/page/year: 15,272,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2690 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
MEPHDN Medical Pharmacy. (Daiichi Seiyaku K.K., 3-14-10 Nihonbashi, Chuo-ku, Tokyo 103, Japan) V.1- 1966- Volume(issue)/page/year: 15,272,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
55 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #4051141
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NDADD8 New Drugs Annual: Cardiovascular Drugs. (New York, NY) V.1-2, 1983-84. For publisher information, see NCDREP. Volume(issue)/page/year: 1,295,1983 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
17400 mg/kg/29D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - other changes in urine composition Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 18,781,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
27375 mg/kg/1Y-I
-
TOXIC EFFECTS :
-
Liver - other changes Liver - changes in liver weight
-
REFERENCE :
-
YKYUA6 Yakkyoku. Pharmacy. (Nanzando, 4-1-11, Yushima, Bunkyo-ku, Tokyo, Japan) V.1- 1950- Volume(issue)/page/year: 32,1499,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4500 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Liver - fatty liver degeneration Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - other changes in urine composition
-
REFERENCE :
-
YKYUA6 Yakkyoku. Pharmacy. (Nanzando, 4-1-11, Yushima, Bunkyo-ku, Tokyo, Japan) V.1- 1950- Volume(issue)/page/year: 32,1499,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
46800 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Endocrine - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 18,797,1979 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10800 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 11,276,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
220 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 11,265,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4400 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 11,265,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
220 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 11,265,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
650 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 11,287,1980
|

![4,5,6,7-Tetrahydrothieno[3,2-c]pyridine structure](https://image.chemsrc.com/caspic/421/54903-50-3.png)
![N-[(2-chlorophenyl)methyl]-2-thiophen-2-ylethanamine,hydrochloride structure](https://image.chemsrc.com/caspic/013/60612-23-9.png)

![N-[(2-chlorophenyl)methyl]-2-thiophen-2-ylethanamine structure](https://image.chemsrc.com/caspic/116/69061-17-2.png)