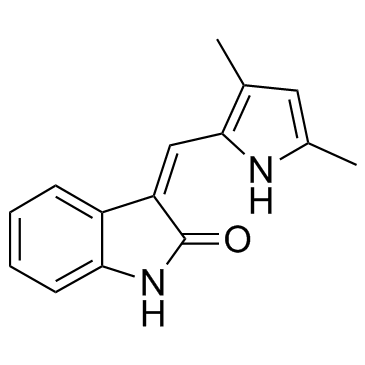
204005-46-9
| Name | 3-[(2,4-dimethylpyrrol-5-yl)methylidenyl]-indolin-2-one |
|---|---|
| Synonyms |
1,3-Dihydro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-2H-indol-2-one
semaxanib (3Z)-3-[(3,5-Dimethyl-1H-pyrrol-2-yl)methylene]-1,3-dihydro-2H-indol-2-one 2H-Indol-2-one, 3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-1,3-dihydro-, (3Z)- SU 5416 SU5416 |
| Description | Semaxinib (SU5416) is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) with an IC50 of 1.23 μM. |
|---|---|
| Related Catalog | |
| Target |
Flk-1:1.23 μM (IC50) |
| In Vitro | Semaxinib (SU5416) inhibits VEGF-driven mitogenesis in a dose-dependent manner with an IC50 of 0.04±0.02 μM (n=3). In contrast, Semaxinib (SU5416) blocked FGF-dependent mitogenesis of HUVECs with an IC50 of 50 μM (n=10). The selective activity of Semaxinib (SU5416) on Flk-1 is supported by the fact that testing of Semaxinib (SU5416) using NIH 3T3 cells overexpressing either the EGF or insulin receptors indicated a complete lack of activity (IC50>100 μM). This observation is confirmed by immunoblotting after ligand stimulation. An IC50 of 20.26±5.2 μM (n=7), which is about 20-fold less in potency on PDGF-dependent autophosphorylation, is observed when SU5416 is tested in NIH 3T3 cells overexpressing the human PDGF receptor β[1]. |
| In Vivo | Daily administration of Semaxinib (SU5416) (i.p., 3 mg/kg/day) inhibits the local growth of C6 tumors in the colon. A comparable level of growth inhibition (62% by day 16; P=0.001) is observed for tumors growing in the colon in comparison with ones growing in the hindflank region (54% by day 18; P=0.001). These results indicate that Semaxinib (SU5416) could inhibit tumor growth at a site other than the subcutaneous implantation site, where the preexisting vasculature may be different[1]. Daily treatment with Semaxinib (SU5416) (25 mg/kg) results in a significantly lower tumor growth rate with tumor masses of up to 8% of that present in control animals by day 22 after implantation. Inhibition of tumor growth is clearly preceded by a marked reduction of the tissue area covered by the newly formed glioma microvasculature in the Semaxinib-treated group, indicating a reduced initial tumor vascularization[2]. |
| Cell Assay | 3T3Her2 and 488G2M2 are NIH3T3 fibroblast cell lines engineered to overexpress Her2 and to express human PDGF-BB and human PDGF receptor β. Both cell lines are cultured in DMEM supplemented with 2% CS and 2 mM L-glutamine. C6, Calu 6, A375, A431, and SF767T are plated in their respective growth medium at 2×103 cells/100 μL/well in 96-well, flat-bottomed plates. Semaxinib (SU5416) is serially diluted in media containing DMSO (<0.5%) and added to cultures of tumor cells 1 day after the initiation of culture. Cell growth is measured after 96 h using the sulforhodamine B method. IC50s are calculated by curve fitting using four-parameter analysis[1]. |
| Animal Admin | Mice[1] Female BALB/c nu/nu mice (20-22 g; 12 weeks of age) are anesthesized using a mixture of xylazine (5 mg/kg) and ketamine (100 mg/kg). Aseptic technique is used during this surgical procedure. A small midline incision (1 cm) is made in the abdomen directly over the colon. C6 cells are implanted (0.5×106 cells/animal) under the serosa of the colon using a 27-gauge needle. After implantation, all exposed sections of the intestine are returned into the abdominal cavity. The peritoneum and skin are closed using a 6.0 surgical suture and wound clips. The wound clips are removed 7 days after surgery. Animals are treated once daily with a 50 μL i.p. bolus injection of either Semaxinib (SU5416) or DMSO, beginning 1 day after implantation. Approximately 13-16 days after implantation, animals are euthanized, and local tumor growth on the colon is quantitated either by weighing the tumors or by measuring the tumors using venier calipers. Tumor volumes are calculated as the product of length×width×height. Rats[3] Male Sprague Dawley rats (n=60, 6-8 wk) are randomly divided among five groups: control (Con), Semaxinib (SU), pneumonectomy (PNx), Semaxinib+hypoxia (SuHx), and Semaxinib+PNx (SuPNx). The SuHx protocol is employed. Briefly, animals are injected with Semaxinib (25 mg/kg) dissolved in carboxymethylcellulose (CMC) and exposed to hypoxia (10%) for 4 wk followed by re-exposure to normoxia. PNx animals underwent a left pneumonectomy. Two days following PNx surgery an injection of Semaxinib is administered (25 mg/kg). The Con group received only the solvent CMC. Echocardiography is utilized at baseline (prehypoxia/presurgery), week 2, and week 6 to determine cardiac morphometry and function. Two and six weeks postsurgery/posthypoxia animals are anesthetized and right ventricle (RV) and left ventricle (LV) pressure measurements via catheterization are performed. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.4±45.0 °C at 760 mmHg |
| Molecular Formula | C15H14N2O |
| Molecular Weight | 238.285 |
| Flash Point | 244.9±28.7 °C |
| Exact Mass | 238.110611 |
| PSA | 44.89000 |
| LogP | 2.87 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.684 |
| Storage condition | -20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |