215303-72-3
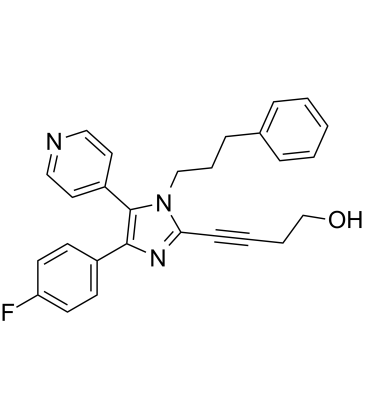
| Name | 4-[4-(4-fluorophenyl)-1-(3-phenylpropyl)-5-pyridin-4-ylimidazol-2-yl]but-3-yn-1-ol |
|---|---|
| Synonyms |
4-(4-(4-Fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridinyl)-1H-imidazol-2-yl)-3-butyn-1-ol
4-[4-(4-Fluorophenyl)-1-(3-phenylpropyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl]but-3-yn-1-ol 3-Butyn-1-ol, 4-(4-(4-fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridinyl)-1H-imidazol-2-yl)- 3-Butyn-1-ol, 4-[4-(4-fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]- 4-[4-(4-Fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]-3-butyn-1-ol 4-(4-Fluorophenyl)-2-(4-hydroxy-1-butynyl)-1-(3-Phenylpropyl)-5-(4-Pyridyl)imidazole RWJ-67657 |
| Description | RWJ 67657 (JNJ 3026582) is an orally active and selective p38α and p38β MAPK inhibitor with IC50s of 1 and 11 μM, respectively. RWJ 67657 displays no activity at p38γ and p38δ, and exhibits cardio protective. Anti-inflammatory and anti-tumor activity[1]. |
|---|---|
| Related Catalog | |
| Target |
p38α:1 μM (IC50) p38β:11 μM (IC50) |
| In Vitro | RWJ 67657 inhibits the release of TNF-α by lipopolysaccharide (LPS)-treated human peripheral blood mononuclear cells with an IC50 of 3 nM, as well as the release of TNF-α from peripheral blood mononuclear cells treated with the superantigen staphylococcal enterotoxin B, with an IC50 value of 13 nM[2]. RWJ67657 (10 μM; 24 hours) decreases colony formation in MCF-7 cells[3]. Cell Proliferation Assay[3] Cell Line: MCF-7 breast carcinoma cells Concentration: 10 μM Incubation Time: 24 hours Result: Did decrease colony formation. |
| In Vivo | RWJ 67657 inhibits TNF-alpha production in lipopolysaccharide-injected mice (87% inhibition at 50 mg/kg) and in rats (91% inhibition at 25 mg/kg) after oral administration[2]. RWJ-67657 (50 mg/kg; administered orally; once per day for 7 consecutive days) displays a potent anti-inflammatory effect. By both improving the functioning of endothelial progenitor cells (EPCs) and reducing inflammation, EPC transplantation plus RWJ-67657 administration synergistically promotes angiogenesis and neurogenesis after diabetic stroke[4]. Animal Model: db/db mice (male, 8 weeks old) with EPCs[4] Dosage: 50 mg/kg Administration: Administered orally; once per day for 7 consecutive days Result: Increased angiogenesis and neurogenesis of diabetic mice after cotreatment with EPCs transplantation. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 611.8±65.0 °C at 760 mmHg |
| Melting Point | 124℃ |
| Molecular Formula | C27H24FN3O |
| Molecular Weight | 425.497 |
| Flash Point | 323.8±34.3 °C |
| Exact Mass | 425.190338 |
| PSA | 50.94000 |
| LogP | 5.18 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.599 |
| Storage condition | Store at RT |