479-41-4
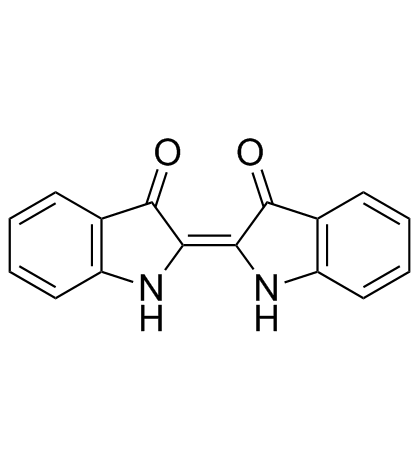
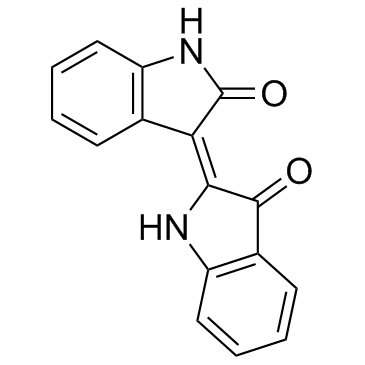
| Name | Indirubin |
|---|---|
| Synonyms |
Indirubin
(3Z)-3-(3-Oxo-1,3-dihydro-2H-indol-2-ylidene)-1,3-dihydro-2H-indol-2-one Indigo Red 2-ylidene)-1,3-dihydro COUROUPITINE B [2,3'-Biindolinylidene]-2',3-dione MFCD00221745 INDIGOPURPURIN 2H-Indol-2-one, 3-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,3-dihydro-, (3Z)- |
| Description | Indirubin(Couroupitine B) is a purple 3,2- bisindole and a stable isomer of indigo isolated from Indigo naturalis (Apiaceae); anti-inflammatory and anticancer activities.IC50 value:Target:in vitro: The activation of EGF receptor, known to be highly expressed in psoriatic lesions, was inhibited by indigo naturalis or indirubin. The cell proliferation and CDC25B expression of epidermal keratinocytes were induced by EGF alone and confirmed to be inhibited by indigo naturalis or indirubin [2]. indirubin inhibited prostate tumor growth through inhibiting tumor angiogenesis. indirubin inhibited angiogenesis in vivo. We also showed the inhibition activity of indirubin in endothelial cell migration, tube formation and cell survival in vitro [3].in vivo: Indirubin treatment suppressed skin inflammation in DNCB-exposed mice. The skin lesions were significantly thinner in the Indirubin-treated group than in untreated controls, and the hyperkeratosis disappeared. Indirubin reduced the total serum IgE level and cytokines production. In addition, it normalized NF-κB, IκB-α and MAP kinase expression [1]. Indirubin dose-dependently inhibited intersegmental vessel formation in zebrafish embryos. It also inhibited HUVEC proliferation by the induction of cellular apoptosis and cell-cycle arrest at the G0/G1 phase [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 496.6±45.0 °C at 760 mmHg |
| Melting Point | 350 °C |
| Molecular Formula | C16H10N2O2 |
| Molecular Weight | 262.263 |
| Flash Point | 207.0±28.9 °C |
| Exact Mass | 262.074219 |
| PSA | 58.20000 |
| LogP | 2.48 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.709 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 10 | |
|---|---|
| DownStream 5 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


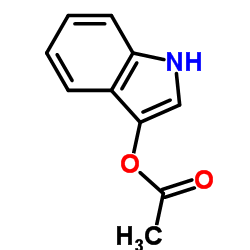
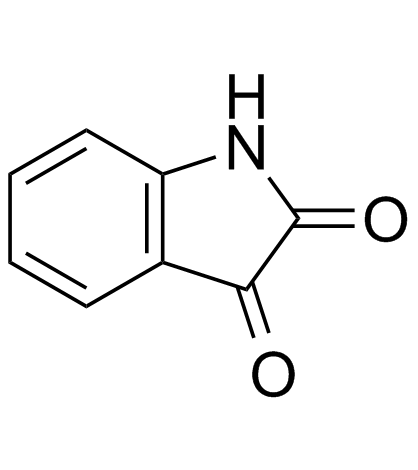
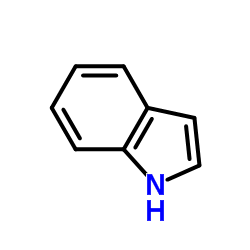
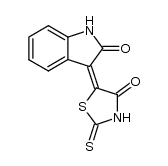

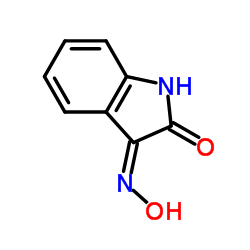
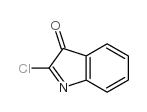
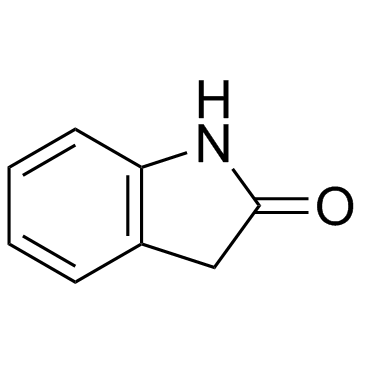
![3'-(2-nitro-benzoyl)-spiro[indolin-3,2'-oxiran]-2-one structure](https://image.chemsrc.com/caspic/209/856068-50-3.png)