CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
HP1626000
-
CHEMICAL NAME :
-
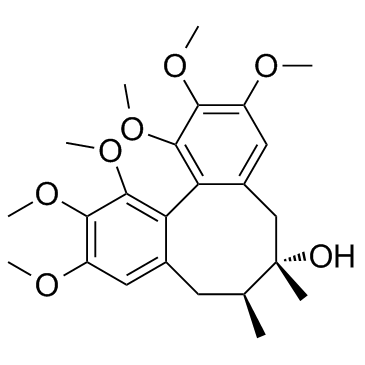
Dibenzo(a,c)cycloocten-6-ol, 5,6,7,8-tetrahydro-6,7-dimethyl-1,2 3,10,11,12-hexamethoxy-, stereoisomer
-
CAS REGISTRY NUMBER :
-
7432-28-2
-
LAST UPDATED :
-
199106
-
DATA ITEMS CITED :
-
3
-
MOLECULAR FORMULA :
-
C24-H32-O7
-
MOLECULAR WEIGHT :
-
432.56
-
WISWESSER LINE NOTATION :
-
L B686&T&J CO1 DO1 EO1 IQ I1 J1 NO1 OO1 PO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1448 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - smooth muscle relaxant (mechanism undefined, spasmolytic) Lungs, Thorax, or Respiration - other changes Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. (Nippon Yakugakkai, 2-12-15 Shibuya, Shibuya-ku, Tokyo 150, Japan) No.1- 1881- Volume(issue)/page/year: 101,1030,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
518 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - smooth muscle relaxant (mechanism undefined, spasmolytic) Lungs, Thorax, or Respiration - other changes Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. (Nippon Yakugakkai, 2-12-15 Shibuya, Shibuya-ku, Tokyo 150, Japan) No.1- 1881- Volume(issue)/page/year: 101,1030,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1861 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - sleep Behavioral - somnolence (general depressed activity) Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. (Nippon Yakugakkai, 2-12-15 Shibuya, Shibuya-ku, Tokyo 150, Japan) No.1- 1881- Volume(issue)/page/year: 101,1030,1981
|