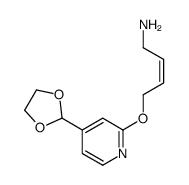
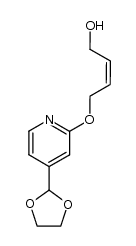
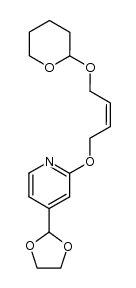
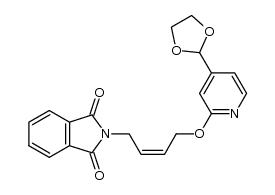
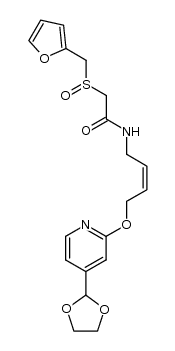
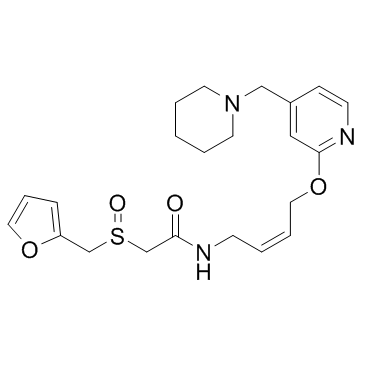
118288-08-7
| Name | Lafutidine |
|---|---|
| Synonyms |
(Z)-2-((2-Furanylmethyl)sulfinyl)-N-(4-((4-(1-piperidinylmethyl)-2-pyridinyl)oxy)-2-butenyl)acetamide
rac-2-(furfurylsulfinyl)-N-[(Z)-4-{[4-(piperidinomethyl)-2-pyridyl]oxy}but-2-enyl]acetamide 2-[(Furan-2-ylmethyl)sulfinyl]-N-[(2Z)-4-{[4-(piperidin-1-ylmethyl)pyridin-2-yl]oxy}but-2-en-1-yl]acetamid (+/-)-2-(furfurylsulfinyl)-N-[4-[4-(piperidinomethyl)-2-pyridyl]oxy-(Z)-2-butenyl]acetamide (z)-inyl)oxy)-2-butenyl) Acetamide, 2-[(2-furanylmethyl)sulfinyl]-N-[(2Z)-4-[[4-(1-piperidinylmethyl)-2-pyridinyl]oxy]-2-buten-1-yl]- LAFUTIDINE(SUBJECTTOPATENTFREE) Protecadin Stogar Laflutidine 2-[(2-Furylmethyl)sulfinyl]-N-[(2Z)-4-{[4-(piperidin-1-ylmethyl)pyridin-2-yl]oxy}but-2-en-1-yl]acetamide 2-[(furan-2-ylmethyl)sulfinyl]-N-[(2Z)-4-{[4-(piperidin-1-ylmethyl)pyridin-2-yl]oxy}but-2-en-1-yl]acetamide lafutidine 2-[(2-Furylmethyl)sulfinyl]-N-[(2Z)-4-{[4-(1-piperidinylmethyl)-2-pyridinyl]oxy}-2-buten-1-yl]acetamide enyl)acetamide MFCD00867520 (±)-2-(Furfurylsulfinyl)-N-(4-(4-(piperidinomethyl)-2-pyridyl)oxy-(Z)-2-butenyl)acetamide |
| Description | Lafutidine, a newly developed histamine H(2)-receptor antagonist, inhibits gastric acid secretion.Target: histamine H(2)-receptorLafutidine, a newly developed histamine H(2)-receptor antagonist, inhibits gastric acid secretion.It is currently marketed in Japan (Stogar) China (Lemeiting) and India (Lafaxid). It not only suppresses gastric acid secretion, but also has cytoprotective properties by the virtue of its property to induce the collagen synthesis in the gastric mucosa. It has a novel mechanism of action in addition to blocking the H2 receptors, it decreases inflammation by modulating calcitonin gene-related peptide (CGRP) and vanilloid receptors. It is also found to stimulate mucin biosynthesis and promote the restitution of damaged mucosa.Lafutidine is absorbed in the small intestine, reaches gastric cells via the systemic circulation, and then directly and rapidly binds to gastric cell histamine H2 receptors, thereby inhibiting the stimulation of cAMP and a resultant decrease in acid production (antisecretory action). It causes a sustained increase in intracellular Ca2+ ion concentration in endothelial cells resulting in the release of Calcitonin Gene Related Peptide (CGRP), which causes acid suppression by decreasing the vagal tone. Lafutidine also increases plasma somatostatin levels which decreases secretion of gastrin from G cells. This decrease in gastrin causes inhibition of parietal cells, resulting in decrease in gastric acid secretion. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 704.2±60.0 °C at 760 mmHg |
| Melting Point | 99 °C |
| Molecular Formula | C22H29N3O4S |
| Molecular Weight | 431.548 |
| Flash Point | 379.7±32.9 °C |
| Exact Mass | 431.187866 |
| PSA | 103.88000 |
| LogP | 1.10 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.599 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36/37/39 |
| HS Code | 2929909090 |
|
~% 
118288-08-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 46, # 4 p. 616 - 622 |
|
~% 
118288-08-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 46, # 4 p. 616 - 622 |
|
~% 
118288-08-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 46, # 4 p. 616 - 622 |
|
~% 
118288-08-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 46, # 4 p. 616 - 622 |
|
~% 
118288-08-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 46, # 4 p. 616 - 622 |
|
~% 
118288-08-7 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 46, # 4 p. 616 - 622 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |