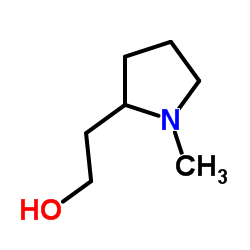
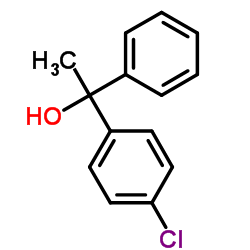
14976-57-9
| Name | clemastine fumarate |
|---|---|
| Synonyms |
(+)-2-[2-[(p-Chloro-α-methyl-α-phenylbenzyl)oxy]ethyl]-1-methylpyrrolidine fumarate salt
(2R)-2-{2-[1-(4-Chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine (2E)-2-butenedioate (1:1) acide (2E)-but-2-ènedioïque - (2R)-2-{2-[(1R)-1-(4-chlorophényl)-1-phényléthoxy]éthyl}-1-méthylpyrrolidine (1:1) Pyrrolidine, 2-[2-[1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methyl-, (2R)-, (2E)-2-butenedioate (1:1) (2E)-But-2-endisäure--(2R)-2-{2-[(1R)-1-(4-chlorphenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidin(1:1) Tavist,Agasten MFCD00137486 (2R)-2-{2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine (2E)-but-2-enedioate EINECS 239-055-2 Clemastine fumarate Pyrrolidine (2-[2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methyl Clemastine fumarate salt (+)-(2R)-2-(2-(((R)-p-Chloro-a-methyl-a-phenylbenzyl)oxy)ethyl)-1-methylpyrrolidine Fumarate (1:1) (2R)-2-{2-[(1R)-1-(4-Chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine (2E)-but-2-enedioate (1:1) |
| Description | Clemastine Fumarate is a selective histamine H1 receptor antagonist with IC50 of 3 nM.Target: Histamine H1 ReceptorClemastine Fumarate inhibits histamine induced rise in [Ca2+]i in HL-60 cells with an IC50 of 3 nM as compared with that of chlorpheniramine or diphenhydramine with IC50 values of 20 nM and 100 nM, respectively [1]. Clemastine showed a first-pass reduction in the extent of absorption, with oral bioavailability calculated as 39.2 +/- 12.4%. Extravascular distribution of drug was suggested by the high volume of distribution (799 +/- 315 L) and low Cmax (0.577 +/- 0.252 ng/mL/mg) observed at 4.77 +/- 2.26 hours after administration, and by the biphasic decline in plasma concentration. The terminal elimination half-life (t1/2) of clemastine was 21.3 +/- 11.6 hours. Steady-state concentrations of clemastine were consistent with linear pharmacokinetic processes, and clearance was unaffected by age in the range studied, or by race [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.097 g/cm3 |
|---|---|
| Boiling Point | 116 °C / 24mmHg |
| Melting Point | 61 °C |
| Molecular Formula | C25H30ClNO5 |
| Molecular Weight | 459.962 |
| Flash Point | 211ºC |
| Exact Mass | 459.181244 |
| PSA | 87.07000 |
| LogP | 4.75410 |
| Vapour Pressure | 1.94E-07mmHg at 25°C |
| Index of Refraction | 1.553 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | UY0704600 |
| HS Code | 2933990090 |
| Precursor 0 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |