112887-68-0
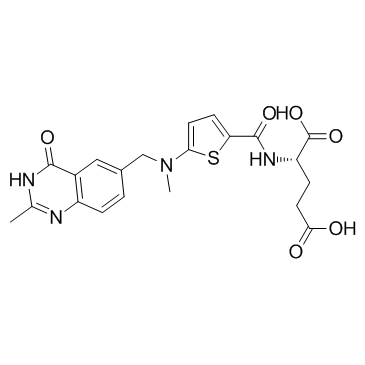
| Name | (2S)-2-[[5-[methyl-[(2-methyl-4-oxo-1H-quinazolin-6-yl)methyl]amino]thiophene-2-carbonyl]amino]pentanedioic acid |
|---|---|
| Synonyms |
N-[5-[N-[(3,4-dihydro-2-methyl-4-oxy-6-quinazolinyl)-methyl]-N-methylamino]-2-thenoyl]-L-glutamic acid
2kce ZD1694 L-Glutamic acid, N-[[5-[[(1,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]methylamino]-2-thienyl]carbonyl]- N-[(5-{methyl[(2-methyl-4-oxo-3,4-dihydroquinazolin-6-yl)methyl]amino}thiophen-2-yl)carbonyl]-L-glutamic acid N-[(5-{Methyl[(2-methyl-4-oxo-3,4-dihydroquinazolin-6-yl)methyl]amino}-2-thienyl)carbonyl]-L-glutamic acid Raltitrexed D1694 ICI-D 1694 MFCD00864168 N-(5-[N-(3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl)-N-methylamino]-2-thenoyl)-L-glutamic acid Tomudex 2tsr N-[[5-[[(1,4-Dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]methylamino]-2-thienyl]carbonyl]-L-glutamic acid ICI D1694 N-[(5-{Methyl[(2-methyl-4-oxo-1,4-dihydro-6-quinazolinyl)methyl]amino}-2-thienyl)carbonyl]-L-glutamic acid |
| Description | Raltitrexed is an antimetabolite drug used in chemotherapy, acting by inhibiting thymidylate synthase. |
|---|---|
| Related Catalog | |
| In Vitro | Raltitrexed inhibits HepG2 proliferation by arresting the cell cycle at G0/G1, and the cell cycle is mediated via downregulation of cyclin A and CDK2[1]. Raltitrexed (0.1, 0.5, 2.5 μg/mL) decreases the viability of SGC7901 cells in a dose- and time-dependent manner. Raltitrexed (0.5 μg/mL) shows typical apoptotic morphology, including nuclear shrinkage, fragmentation, chromatin condensation and apoptotic bodies in SGC7901 cells. Raltitrexed blocks the cell cycle at the G0/G1 phase, decreases in the mitochondrial membrane potential. Raltitrexed also increases the level of ROS, induces caspase-3-dependent apoptosis via activation of the mitochondria, and increases TS protein and mRNA expression levels[3]. Raltitrexed (1.5 nM) reduces the number of GM00637 cells, selectively induces gene conversions, but does not affect DSB-induced HR or NHEJ[4]. |
| In Vivo | Raltitrexed (0, 5, 10, 11.5, 13.5, 15 mg/kg b/w, i.p.) increases the rates of resorbed embryos and growth retardation of murine model of NTDs in a dose dependent manner. Raltitrexed (11.5 mg/kg b/w) maximally inhibits the thymidylate synthase (TS) activity in embryonic tissue, decreases dTMP levels and while increases dUMP levels[2]. |
| Cell Assay | To assess the effect of Raltitrexed on cell viability and/or growth, GM00637 cells are plated into 25 cm2 flasks at a density of 3.3×105 cells per flask. Twenty four hours later, the medium is replaced with medium supplemented with various doses of Raltitrexed over a broad range of concentrations ranging from less than 1 nM to greater than 1 µM. Three flasks of cells are used for each dose tested. Cells are exposed to Raltitrexed for 24 hours, at which time the cells are refed with medium containing no Raltitrexed. Forty-eight hours after feeding with drug-free medium, cells are harvested and counted. The cell counts for the cells exposed to the various Raltitrexed doses are compared with the cell count for control cells not exposed to Raltitrexed as a measure of the impact of Raltitrexed on cell viability and/or growth rate. |
| Animal Admin | The adult (7-8 week, 19-20 g) C57BL/6 mice are used in the experiment. Mice are maintained under 22°C with a 12 h light/day cycle and fed with standard mouse chow and tap water ad libitum. Female mice are mated with male overnight and vaginal plugs are examined in the following morning. The presence of vaginal plug in the pregnant mice is considered as gestational day 0.5. Pregnant mice are randomly divided into 6 groups with 10 mice in each group. Raltitrexed is dissolved in 0.9 % NaCl, and five groups are intraperitoneally injected with different doses of Raltitrexed (5, 10, 11.5, 13.5, 15 mg/kg b/w) on gestational day 7.5. The control group is intraperitoneally injected with 0.9 % NaCl at the same volume on gestational day 7.5. On gestational day 11.5, pregnant mice are sacrificed, and embryos are examined under dissect microscope. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Melting Point | 176-1800C |
| Molecular Formula | C21H22N4O6S |
| Molecular Weight | 458.488 |
| Exact Mass | 458.126007 |
| PSA | 180.93000 |
| LogP | -1.28 |
| Index of Refraction | 1.692 |
| Storage condition | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S36/37/39-S45-S25 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 8 |
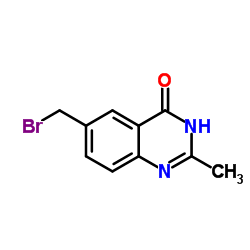
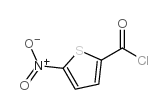
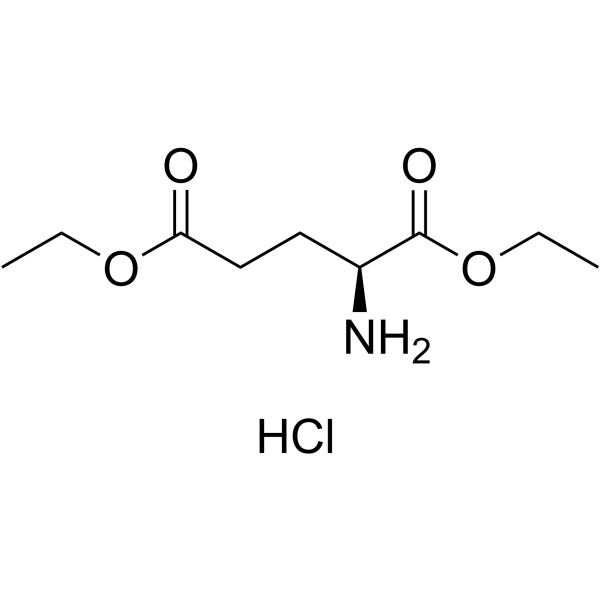
| Precursor 7 | |
|---|---|
| DownStream 0 | |


![Diethyl N-[5-[N-[(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]-N-methylamino]-2-thenoyl]-L-glutamate structure](https://image.chemsrc.com/caspic/067/132463-02-6.png)
![Diethyl N-[5-methylamino-2-thenoyl]-L-glutamate structure](https://image.chemsrc.com/caspic/095/112889-02-8.png)
![5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thiophenecarboxylic acid structure](https://image.chemsrc.com/caspic/457/131052-68-1.png)
