63-92-3
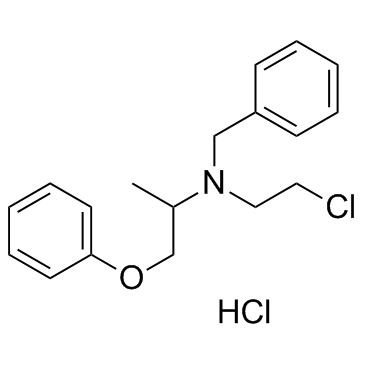
| Name | Phenoxybenzamine hydrochloride |
|---|---|
| Synonyms |
Benzylamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-, hydrochloride
Benzenemethanamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-, hydrochloride (1:1) N-benzyl-N-(2-chloroéthyl)-1-phénoxypropan-2-amine chlorhydrate MFCD00599580 N-benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine,hydrochloride Bensylyte Hydrochloride N-Benzyl-N-(2-chlorethyl)-1-phenoxypropan-2-aminhydrochlorid Dibenyline Benzenemethanamine, N- (2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-, hydrochloride Benzylamine, N- (2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-, hydrochloride Phenoxybenzamine HCl N-(2-Chloroethyl)-N-(1-methyl-2-phenoxyethyl)benzylamine, hydrochloride (±)-Phenoxybenzamine Hydrochloride N-Phenoxyisopropyl-N-benzyl-b-chloroethylamine Hydrochloride N-Benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine hydrochloride (1:1) Bensylyt Hydrochloride Phenoxybenzamine Hydrochloride EINECS 200-569-7 Dibenzyran N-Benzyl-N-(2-chloroethyl)-1-phenoxy-2-propanamine hydrochloride (1:1) Phenoxybenzamine (hydrochloride) |
| Description | Phenoxybenzamine hydrochloride is a selective antagonist of both α-adrenoceptor and calmodulin that is commonly used for the treatment of hypertension, specifically caused by pheochromocytoma. |
|---|---|
| Related Catalog | |
| In Vitro | The IC50 (100 nM) derived from the blockade of [3H]yohimbine binding by Phenoxybenzamine hydrochloride is significantly less than the IC50 (550 nM) for the corresponding reversal by Phenoxybenzamine hydrochloride of the effects of norepinephrine on cyclic AMP accumulation[1]. Phenoxybenzamine hydrochloride (50 nM) in conbination with Phenoxybenzamine hydrochloridetolamine (1000 nM) enhances Phenoxybenzamine hydrochlorideylephrine-induced contraction compared with pretreatment with Phenoxybenzamine hydrochloride (50 nM) alone in endothelium-intact aortae. Combined treatment with either dexmedetomidine (300 or 1000 nM) and Phenoxybenzamine hydrochloride (50 nM) or Phenoxybenzamine hydrochloridetolamine (1000 nM) and Phenoxybenzamine hydrochloride (50 nM) enhance Phenoxybenzamine hydrochlorideylephrine-induced contraction compared with Phenoxybenzamine hydrochloride alone (50 nM). In addition, combined treatment with Phenoxybenzamine hydrochloridetolamine and Phenoxybenzamine hydrochloride enhances Phenoxybenzamine hydrochlorideylephrine-induced contraction compared with dexmedetomidine (1000 nM) and Phenoxybenzamine hydrochloride combined treatment. Combined treatment with high concentrations of dexmedetomidine (1000 nM) and Phenoxybenzamine hydrochloride enhances Phenoxybenzamine hydrochlorideylephrine-induced contraction compared with combined treatment with low concentrations of dexmedetomidine (300 nM) and Phenoxybenzamine hydrochloride[2]. Phenoxybenzamine hydrochloride (0.1-100 μM) inhibits glioma proliferation, migration, and invasion and suppresses the tumorigenesis capacity. Phenoxybenzamine hydrochloride also inhibits self-renewal of glioma stem-like cells. Phenoxybenzamine hydrochloride activates LINGO-1 and inhibits the TrkB-Akt pathway[3]. Phenoxybenzamine hydrochloride (0.1 μM-1 mM) preserves primary neurons within the CA1, CA3 and dentate gyrus and produces a robust neuroprotective effect, and prevents neuronal death from OGD in all regions of the hippocampus when delivered at 2, 4, and 8 h post-OGD at 100 μM[4]. |
| In Vivo | Phenoxybenzamine hydrochloride (20 nM, s.c.) effectively suppresses the tumorigenesis of glioma cells in mice and the cell density in Phenoxybenzamine hydrochloride-U87MG xenografts decreases significantly[3]. Phenoxybenzamine hydrochloride (1 mg/kg, i.v.) treated rats shows significant improvements in NSS and foot fault scoring[4]. |
| Cell Assay | After cytometry, 1×3 cells are implanted in a 96-well plate in 100 μL DMEM supplemented with 10 % FBS. Ten microliter (10 % of the total volume) WST-1 (Water Soluble Tetrazolium) is added to cells and incubated at 37°C for 30 min before colorimetric assay with 450 nm excitation and 630 nm emission at 24 h intervals up to 96 h. The mean fluorescence value is counted, and the cell number is determined using the standard curve. |
| Animal Admin | U87MG cells are injected into both flanks of the nude mice subcutaneously at a dose of 2.0×3/200 μL per side. Eight days after injection, neoplasm growth is observed macroscopically on both sides of the mice. Then, 20 nM phenoxybenzamine hydrochloride is injected into the right side subcutaneously at a 2-day interval, and the dissolvent DMSO is used as control. The tumor volume (V) is determined by measuring the length (a) and the width (b) and calculated using the equation: V=(ab)2/2. |
| References |
| Boiling Point | 381.5ºC at 760 mmHg |
|---|---|
| Melting Point | 137.5°C |
| Molecular Formula | C18H23Cl2NO |
| Molecular Weight | 340.287 |
| Flash Point | 184.5ºC |
| Exact Mass | 339.115662 |
| PSA | 12.47000 |
| LogP | 4.99690 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: slightly soluble | <0.01 g/100 mL at 18.5 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H351 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R40 |
| Safety Phrases | S22-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DP3750000 |
| HS Code | 2922299090 |
| HS Code | 2922299090 |
|---|---|
| Summary | 2922299090. other amino-naphthols and other amino-phenols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |