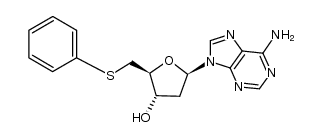
6698-26-6
| Name | 2′,5′-Dideoxyadenosine |
|---|---|
| Synonyms | 2',5'-dideoxyadenosine |
| Description | 2',5'-Dideoxyadenosine is a potent and non-competitive adenylyl cyclase inhibitor via binding the P-site with an IC50 of 3 µM . 2',5'-Dideoxyadenosine is a nucleoside analog and exerts a potent antiadrenergic action in heart[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3 µM (adenylyl cyclase)[1] |
| In Vitro | 2',5'-Dideoxyadenosine (10 μM, 30 min) reduces cAMP production and blocks the phosphorylation of GluA1 at Ser845 induced by carbachol (CCh)[3]. 2',5'-Dideoxyadenosine (10 μM, 30 min) blocks CCh-induced increase of phosphorylation of Akt and attenuates CCh-induced phosphorylation of Ser2448[3]. 2',5'-Dideoxyadenosine (20-150 mM), like adenosine, dependently and reversibly inhibits the positive inotropic and chronotropic effect of beta-adrenergic stimulation with isoproterenol (8-54 pmol) up to 70% and 50%, respectively[2]. Western Blot Analysis[3] Cell Line: Primary hippocampal neurons Concentration: 10 μM Incubation Time: 30 min Result: Reduced cAMP production and blocked the phosphorylation of GluA1 at Ser845 induced by carbachol (CCh). |
| In Vivo | 2',5'-Dideoxyadenosine (0.1 mg/kg; IP; 15 min pre-treated) fully inhibits the diuretic, natriuretic and K+ and Cl- sparing effect of Fr•EtOAc in rats[4]. Animal Model: Male Wistar rats (3-4 months old)[3] Dosage: 0.1 mg/kg Administration: IP; 15 min pre-treated Result: Fully inhibited the diuretic, natriuretic and K+ and Cl- sparing effect of Fr•EtOAc in rats. |
| References |
| Density | 1.77 g/cm3 |
|---|---|
| Boiling Point | 547ºC at 760 mmHg |
| Molecular Formula | C10H13N5O2 |
| Molecular Weight | 235.24300 |
| Flash Point | 284.6ºC |
| Exact Mass | 235.10700 |
| PSA | 99.08000 |
| LogP | 0.65800 |
| Index of Refraction | 1.825 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
|
~72% 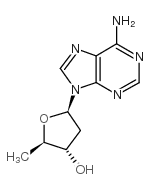
6698-26-6 |
| Literature: Desaubry; Shoshani; Johnson Nucleosides and Nucleotides, 1995 , vol. 14, # 6 p. 1453 - 1460 |
|
~% 
6698-26-6 |
| Literature: Nucleosides and Nucleotides, , vol. 14, # 6 p. 1453 - 1460 |
| Precursor 2 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |