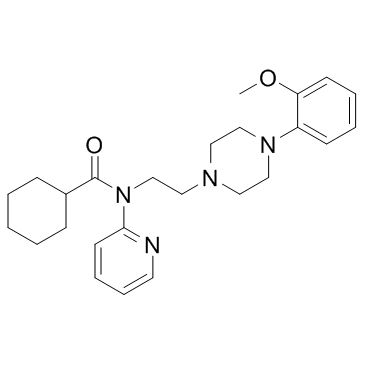
162760-96-5
| Name | N-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-pyridin-2-ylcyclohexanecarboxamide |
|---|---|
| Synonyms |
Lopac-W-108
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)-cyclo-hexanecarboxamide N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate cyclohexanecarboxamide,n-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-n-2-pyridinyl N-{2-[4-(methoxyphenyl)-1-piperazinyl]ethyl}-N-(2-pyridinyl)-cyclohexanecarboxamide WAY-100635 |
| Description | WAY-100635 is a potent and selective 5-HT1A Receptor antagonist with a pIC50 of 8.87, an apparent pA2 of 9.71. |
|---|---|
| Related Catalog | |
| Target |
5-HT1A Receptor:8.87 (pIC50) 5-HT1A Receptor:9.71 (pA2) |
| In Vitro | WAY-100635 is a potent and, at high concentrations, an insurmountable antagonist of the 5-HT1A receptor agonist action of 5-carboxamidotryptamine, with an apparent pA2 value (at 0.3 nM) of 9.71. WAY-100635 displaces specific binding of the 5-HT1A radioligand, [3H]8-OH-DPAT (8-hydroxy-2-(di-n-propylamino)tetralin), to rat hippocampal membranes with a plCIC50 of 8.87[1]. |
| In Vivo | Administration of (S)-WAY-100135 (0.025-1.0 mg/kg i.v.) moderately depresses neuronal activity at all doses tested. In contrast, administration of WAY-100635 (0.025-0.5 mg/kg i.v.) significantly increases neuronal activity. The stimulatory action of WAY-100635, like that of spiperone, is evident during wakefulness but not during sleep. Pretreatment with (S)-WAY-100135 (0.5 mg/kg i.v.) weakly attenuates the inhibitory action of 8-hydroxy-2-(di-n-propylamino) tetralin. In contrast, WAY-100635 at doses as low as 0.1 mg/kg i.v. completely blockes the action of 8-hydroxy-2-(di-n-propylamino) tetralin. The antagonist action of WAY-100635 at 5-HT1A autoreceptors closely parallels its ability to increase neuronal activity. Overall, WAY-100635 appears to act as a selective 5-HT1A antagonist, whereas (S)-WAY-100135 does not. The results obtained with WAY-100635 confirm our previous findings obtained with spiperone and further support the hypothesis that 5-HT1A autoreceptor-mediated feedback inhibition operates under physiological conditions[2]. |
| References |
| Molecular Formula | C25H34N4O2 |
|---|---|
| Molecular Weight | 422.56300 |
| Exact Mass | 422.26800 |
| PSA | 48.91000 |
| LogP | 3.82860 |
| Storage condition | -20°C |
| Precursor 7 | |
|---|---|
| DownStream 0 | |