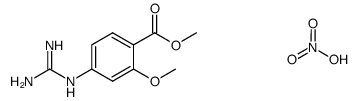
1028486-01-2
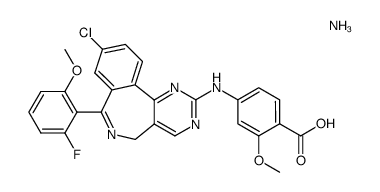
| Name | 4-[[9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino]-2-methoxybenzoic acid |
|---|---|
| Synonyms |
4-{[9-Chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino}-2-methoxybenzoic acid
Alisertib MLN 8237 4-{[9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino}-2-methoxy-benzoic acid MLN8237 |
| Description | Alisertib (MLN 8237) is a selective Aurora A inhibitor with an IC50 of 1.2 nM. |
|---|---|
| Related Catalog | |
| Target |
Aurora A:1.2 nM (IC50) |
| In Vitro | Alisertib leads the MM cells to mitotic spindle abnormalities, mitotic accumulation, as well as inhibition of cell proliferation through apoptosis and senescence. Alisertib up-regulates p53 and tumor suppressor genes p21 and p27[1]. The decreased activity of MLN8054/Alisertib for the T217D/W277E Aurora A/TPX2 complex may reflect the increased affinity for ATP induced by cofactor binding to Aurora A[2]. Alisertib inhibits cell proliferation with IC50 values ranging from 15 to 469 nM in different tumer cell lines[3]. |
| In Vivo | Alisertib (Alisertib, 30 mg/kg, p.o.) significantly reduces tumor burden and increases overall survival in xenograft-murine model of human-MM[1]. Alisertib (20, 30 mg/kg, p.o.) causes tumor growth inhibition in solid tumor xenograft models and regressions in in vivo models of lymphoma, and reduces FLT uptake in HCT-116 xenograft tumors[3]. |
| Kinase Assay | To measure Aurora A activity, 25 ng (12.5 mM final concentration) or 250 ng (125 nM final concentration) of purified bacterially expressed Aurora A is assayed in the presence of the appropriate inhibitors (MLN8054, Alisertib), using Histone H3 as substrate for 20 min at 30°C in the presence of 100 μM [γ-32P] ATP. For Aurora A/TPX2 assays, 50 ng of a TPX2 [1-43] peptide, representing a 2-fold molar excess over Aurora A, is included. The Aurora A/TPX2 complex is preformed in kinase reactions prior to subsequent addition of inhibitors and ATP. |
| Cell Assay | MM cell lines are incubated with DMSO or Alisertib (0.125-0.5 μM) in combination with conventional anti-MM agents melphalan (2.5-5 μM), doxorubicin (50-100 nM), or dexamethasone (50-100 nM); and with novel anti-MM agents bortezomib (2.5-5 nM) or lenalidomide (0.5-1 μM) for 72 hours. Cell viability is measured by MTT assay. The combination index (CI) is determined by isobologram analysis using CalcuSyn software, Version 2.0 (CI < 1 indicates synergistic effect; CI=1, additive effect; and CI > 1, no significant combination effect). |
| Animal Admin | Mice are irradiated (200 cGy), and then 5×106 MM1.S cells are inoculated subcutaneously in the right flank. When tumor growth is measurable (2 weeks after the injection), mice are assigned into 4 groups (10 mice each) receiving vehicle orally (100 μL of 10% 2-hydroxypropyl-β-cyclodextrin/1% sodium bicarbonate) or Alisertib (7.5 mg/kg, 15 mg/kg, and 30 mg/kg in a final formulation in 10% 2-hydroxypropyl-β-cyclodextrin/1% sodium bicarbonate) for 21 consecutive days. The maximal tolerated dose of Alisertib in most mouse strains (continuous dosing for 21 days) is approximately 20 mg/kg twice a day (40 mg/kg per day). Tumor volumes are measured by a Vernier caliper every alternate day and calculated using the following formula: length×width2×0.5. Mice are killed at the end of the treatment, 2 hours after the last treatment, or when tumor reaches 2 cm3; tumors are immediately collected from mice and evaluated for induction of apoptosis and cell death by TdT-mediated dUTP nick end labeling (TUNEL) assay. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 729.1±70.0 °C at 760 mmHg |
| Molecular Formula | C27H20ClFN4O4 |
| Molecular Weight | 518.924 |
| Flash Point | 394.8±35.7 °C |
| Exact Mass | 518.115723 |
| PSA | 105.93000 |
| LogP | 5.56 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.671 |
| Storage condition | -20°C |
|
~82% 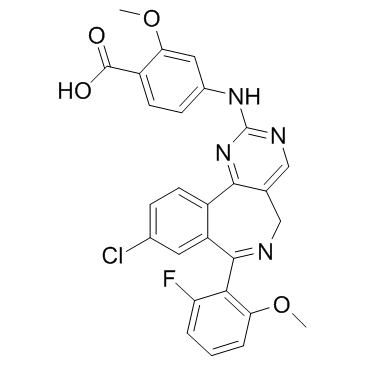
1028486-01-2 |
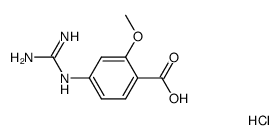
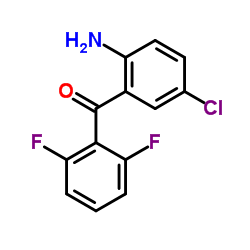
| Literature: MILLENNIUM PHARMACEUTICALS, INC.; ARMITAGE, Ian; COOPER, Martin, I.; EDDLESTON, Mark, D.; FAIBER, Neil, C.; MCCUBBIN, Quentin, J.; WATT, Stephen, W. Patent: WO2011/103089 A1, 2011 ; Location in patent: Page/Page column 45 ; WO 2011/103089 A1 |
|
~% 
1028486-01-2 |
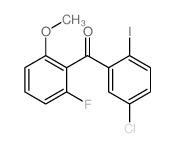
| Literature: WO2008/63525 A1, ; Page/Page column 29-30 ; WO 2008/063525 A1 |
|
~% 
1028486-01-2 |
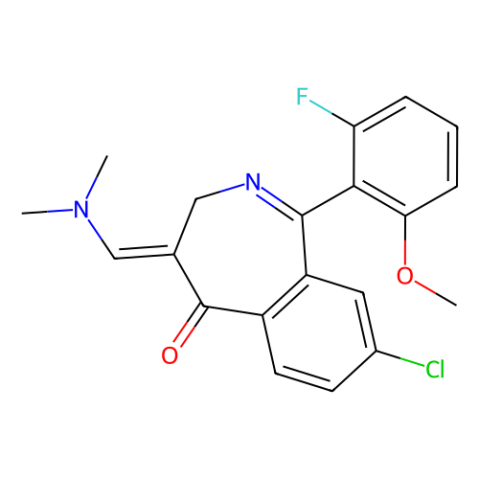
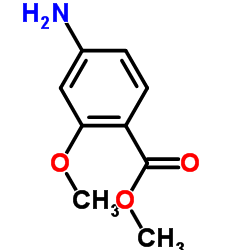
| Literature: WO2011/103089 A1, ; WO 2011/103089 A1 |
|
~% 
1028486-01-2 |
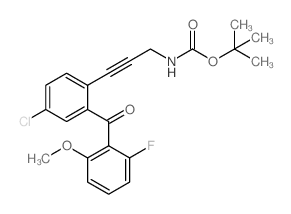
| Literature: WO2011/103089 A1, ; WO 2011/103089 A1 |
|
~% 
1028486-01-2 |
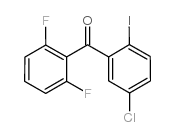
| Literature: WO2011/103089 A1, ; WO 2011/103089 A1 |
|
~% 
1028486-01-2 |
| Literature: WO2011/103089 A1, ; WO 2011/103089 A1 |
|
~% 
1028486-01-2 |
| Literature: WO2011/103089 A1, ; WO 2011/103089 A1 |
|
~% 
1028486-01-2 |
| Literature: WO2011/103089 A1, ; WO 2011/103089 A1 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |