602-41-5
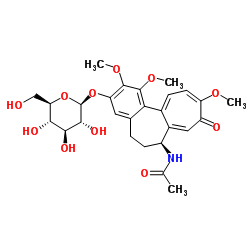
| Name | Thiocolchicoside |
|---|---|
| Synonyms |
Thiocolchicoside
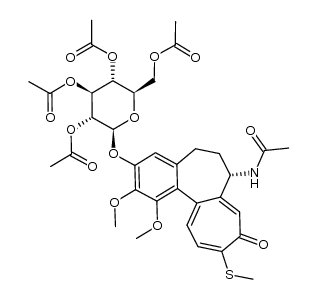
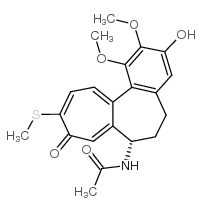
Acetamide, N-[(7S)-3-(β-D-glucopyranosyloxy)-5,6,7,9-tetrahydro-1,2-dimethoxy-10-(methylthio)-9-oxobenzo[a]heptalen-7-yl]- UNII:T1X8S697GT Thiocolchicine Glycoside N-[(7S)-3-(b-D-glucopyranosyloxy)-1,2-dimethoxy-10-(methylthio)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide R. 271 Coltramyl Musco-ril N-[(7S)-3-(β-D-Glucopyranosyloxy)-1,2-dimethoxy-10-(methylsulfanyl)-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide 3,10-Di(demethoxy)-3-glucosyloxy-10-methylthiocolchicine 10-Thiocolchicoside Tiocolchicoside |
| Description | Thiocolchicoside is a competitive γ-aminobutyric acid type A (GABAA) receptor antagonist and glycine receptor agonist in the central nervous system. Thiocolchicoside is a semisynthetic sulfur derivative of colchicoside. Thiocolchicoside is a muscle relaxant and has anti-inflammatory, and analgesic properties[1]. |
|---|---|
| Related Catalog | |
| Target |
GABAA) receptor; glycine receptor[1] |
| In Vitro | Thiocolchicoside (0.001, 0.1, 1, 10, 100 μM) reduces the amplitude of eIPSCs in a concentration-dependent manner with this effect being significant at 0.1 μM and maximal at 10 μM[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 929.6±65.0 °C at 760 mmHg |
| Melting Point | 190-198ºC |
| Molecular Formula | C27H33NO10S |
| Molecular Weight | 563.617 |
| Flash Point | 516.0±34.3 °C |
| Exact Mass | 563.182495 |
| PSA | 189.31000 |
| LogP | -1.23 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.657 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P301 + P312 + P330-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
| RTECS | AC2976015 |
|
~96% 
602-41-5 |
| Literature: Gelmi, Maria Luisa; Fontana, Gabriele; Pocar, Donato; Pontremoli, Guido; Pellegrino, Sara; Bombardelli, Ezio; Riva, Antonella; Balduini, Walter; Carloni, Silvia; Cimino, Mauro Journal of Medicinal Chemistry, 2007 , vol. 50, # 9 p. 2245 - 2248 |
|
~% 
602-41-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2245 - 2248 |
|
~% 
602-41-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 50, # 9 p. 2245 - 2248 |
|
~% 
602-41-5 |
| Literature: Bulletin de la Societe Chimique de France, , p. 198 |
|
~% 
602-41-5 |
| Literature: Bulletin de la Societe Chimique de France, , p. 198 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |