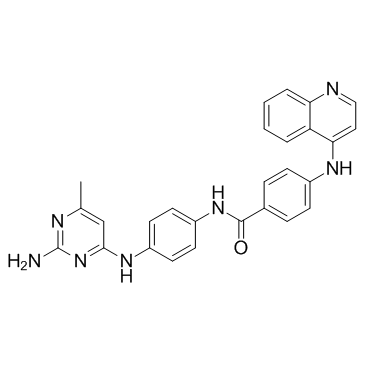
1020149-73-8
| Name | N-{4-[(2-Amino-6-methyl-4-pyrimidinyl)amino]phenyl}-4-(4-quinolin ylamino)benzamide |
|---|---|
| Synonyms |
Benzamide, N-[4-[(2-amino-6-methyl-4-pyrimidinyl)amino]phenyl]-4-(4-quinolinylamino)-
N-{4-[(2-Amino-6-methyl-4-pyrimidinyl)amino]phenyl}-4-(4-quinolinylamino)benzamide SGI-1027 |
| Description | SGI-1027 is a DNA methyltransferase (DNMT) inhibitor, with IC50s of 7.5 μM, 8 μM, and 12.5 μM for DNMT3B, DNMT3A, and DNMT1 with poly(dI-dC) as substrate. |
|---|---|
| Related Catalog | |
| Target |
DNMT3B:7.5 μM (IC50) DNMT3A:8 μM (IC50) DNMT1:12.5 μM (IC50) |
| In Vitro | SGI-1027 is a DNMT inhibitor, with IC50s of 7.5 μM, 8 μM, and 12.5 μM for DNMT3B, DNMT3A, and DNMT1 with poly(dI-dC) as substrate. SGI-1027 shows an IC50 of 6 μM for DNMT1 (hemimethylated DNA). SGI-1027 (1, 2.5, or 5 µM) causes selective degradation of DNMT1 in several human cancer cell lines, but shows little or no cytotoxic effect on rat hepatoma cells, and does not induce apoptosis in rat hepatoma cells[1]. SGI-1027 shows an EC50 of 0.9 μM for hDNMT3A, and causes cytotoxicity on KG-1 cells, with an EC50 of 4.4 μM[2]. |
| Kinase Assay | To determine the nature of inhibition of DNMTase activity by SGI-1027, DNMT1 enzyme activity is measured in presence of a fixed concentration of SGI-1027 (0, 2.5, 5, and 10 μM) while one of the two (Ado-Met or DNA) is varied in a particular reaction mixture. At a fixed concentration of DNA (500 ng) varying concentrations of Ado-Met used are from 25-500 nM, respectively. Similarly, final DNA concentrations are varied from (25-500 ng) at 75 nM Ado-Met[1]. |
| Cell Assay | Rat hepatoma H4IIE cells are grown in DMEM supplemented with fetal bovine serum (10%) and calf serum (10%). Cells are seeded into 96-well plates and after 48 h exposed to SGI-1027 at concentrations ranging from 0 to 300 µM. The solubility is determined by Nephalometry techniques immediately after dosing and before harvesting the cells at 24 h. Following the exposure period, the cells or their supernatant (culture medium) are analyzed for changes in cell proliferation (propidium iodide), membrane leakage (α-GST), mitochondrial function [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and cellular ATP], oxidative stress (intracellular GSH and 8-isoprostane), and apoptosis (caspase-3). The half-maximal toxic concentration (TC50) is determined from the dose-response curves[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | >280℃ |
| Molecular Formula | C27H23N7O |
| Molecular Weight | 461.518 |
| Exact Mass | 461.196411 |
| PSA | 125.30000 |
| LogP | 4.52 |
| Index of Refraction | 1.789 |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| RIDADR | NONH for all modes of transport |