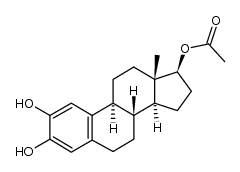
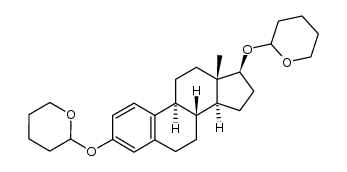
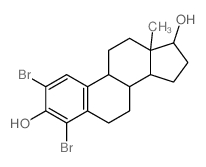
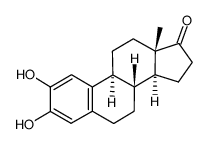
362-05-0
| Name | 2-hydroxy-17β-estradiol |
|---|---|
| Synonyms |
Estra-1,3,5(10)-triene-2,3,17β-triol
2-Hydroxy-17b-estradiol 2-hydroxy-17β-estradiol 2-hydroxy-17beta-estradiol MFCD00010490 (17β)-Estra-1(10),2,4-triene-2,3,17-triol Estra-1,3,5(10)-triene-2,3,17-triol, (17β)- 2-Hydroxyestradiol 2,3,17b-Trihydroxyestra-1,3,5(10)-triene estra-1(10),2,4-triene-2,3,17-triol, (17β)- Estra-1,3,5(10)-triene-2,3,17-β-triol (17b)-Estra-1(10),2,4-triene-2,3,17-triol (17β)-Estra-1,3,5(10)-triene-2,3,17-triol 2-hydroxy-estradiol 2-Oh-estradiol Estra-1,3,5(10)-triene-2,3,17b-triol 2-Hydroxy-17-estradiol |
| Description | 2-Hydroxyestradiol, a metabolite of 17β-estradiol with minimal estrogenic activity, possesses antioxidant effects and reacts with DNA to form stable adducts and exerts genotoxicity[1][3]. |
|---|---|
| Related Catalog | |
| In Vitro | 2-Hydroxyestradiol induces phenotypical changes indicative of neoplastic transformation[1]. 2-Hydroxyestradiol (2-OHE2) is capable of in vitro transforming a HBEC, MCF-10F cell line, that is estrogen receptor-a (ERα) negative and ERβ positive[1]. 2-Hydroxyestradiol induces oxidative DNA damage and apoptosis in human mammary epithelial cells[3]. |
| In Vivo | 2-Hydroxyestradiol attenuated the development of obesity and improved endothelial function, decreased nephropathy, decreased the severity of diabetes, lowered arterial blood pressure, and reduced plasma cholesterol[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.5±45.0 °C at 760 mmHg |
| Melting Point | 96-104°C |
| Molecular Formula | C18H24O3 |
| Molecular Weight | 288.38 |
| Flash Point | 227.6±23.3 °C |
| Exact Mass | 288.172546 |
| PSA | 60.69000 |
| LogP | 3.53 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.622 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 40 |
| Safety Phrases | S22;S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | KG7675000 |
| Precursor 6 | |
|---|---|
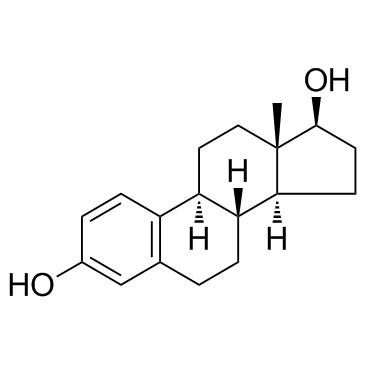
| DownStream 4 | |
![3-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-4,17-diol structure](https://image.chemsrc.com/caspic/360/5976-65-8.png)