1192500-31-4
| Name | avibactam |
|---|---|
| Synonyms |
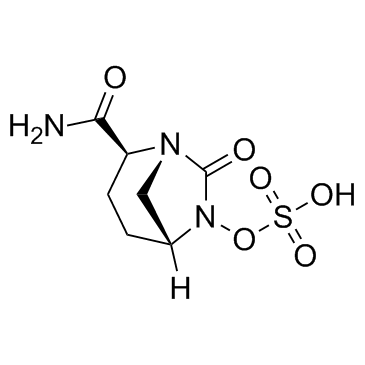
(2S,5R)-7-Oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
1,6-Diazabicyclo[3.2.1]octane-2-carboxamide, 7-oxo-6-(sulfooxy)-, (2S,5R)- (E)-7-fluoro-3-methylhept-4-en-2-one (2S,5R)-7-Oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carbox amide 4-Hepten-2-one,7-fluoro-3-methyl-,(E) trans-7-fluoro-3-methyl-4-hepten-2-one Avibactam (1R,2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl hydrogen sulfate trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide Sulfuric acid, mono[(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl] ester Avibactam (free acid) |
| Description | Avibactam free acid is a covalent, reversible β-lactamase inhibitor, inhibits β-lactamase TEM-1 and CTX-M-15 with IC50 of 8 nM and 5 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 8 nM (TEM-1), 5 nM (CTX-M-15)[1] |
| In Vitro | Avibactam (NXL104) is a molecule with little antibacterial activity, that inhibits class A and C β-lactamases. Avibactam inactivates most important β-lactamases except metallo types and Acinetobacter OXA carbapenemases[2]. |
| In Vivo | Avibactam sodium displays a slow return of activity with an off-rate of 0.045±0.022 min-1, which converts to a residence time half-life (tt1/2) of 16±8 min. The measured off-rate for Avibactam suggests that slow deacylation through hydrolysis or reversibility is occurring, and it is in contrast to previously reported extremely long t1/2 values of >1 or >7 d for Avibactam inhibition of TEM-1[1]. Avibactam is a new promising β-lactamase inhibitor, to overcome resistance caused by β-lactamases. Mice are infected with ca.106 CFU of Pseudomonas aeruginosa intramuscularly into the thigh or intranasally to cause pneumonia and are given 8 different (single) subcutaneous doses of Ceftazidime and Avibactam in various combined concentrations, ranging from 1 to 128 mg/kg of body weight in 2-fold increases. The mean estimated half-life in plasma of Ceftazidime in the terminal phase is 0.28 h (SD, 0.02 h), and that of Avibactam is 0.24 h (SD, 0.04 h). Volumes of distribution are 0.80 liters/kg (SD, 0.14 liters/kg) and 1.18 liters/kg (SD, 0.34 liters/kg), respectively[3]. |
| Kinase Assay | In a 200 μL reaction volume, 1 μM TEM-1 is incubated with and without 5 μM Avibactam for 5 min at 37°C and subjected to two ultrafiltration cartridge (UFC) steps to remove excess inhibitor (Ultrafree-0.5 with Biomax membrane, 5-kDa cutoff). Centrifugation at 10,600× g for 8 min is performed at 4°C. After each ultrafiltration step, 20 μL retentate are diluted with 180 μL assay buffer to restore the original enzyme concentration. After two UFC treatments, the amount of free Avibactam is quantified by liquid chromotography/MS/MS and found to be <5% of the original concentration. Loss of protein during UFC is assessed by measuring TEM-1 activity (on 4,000-fold dilution) in the acyl-enzyme sample compare with non-UFC-treated enzyme, and loss is found to be <5%[1]. |
| Cell Assay | Cells (~109 cfu) from overnight broth culture are spread on Mueller-Hinton agar supplemented with either (i) Ceftaroline plus Avibactam (1 or 4 mg/L) at 1-16× the MICs or (ii) Ceftaroline at 1 or 4 mg/L plus Avibactam at 1-8× the concentration needed to reduce the Ceftaroline MIC to 1 or 4 mg/L. Colonies are counted after overnight incubation and representatives are retained[2]. |
| Animal Admin | Mice[3] Avibactam is reconstituted in sterile water to a stock solution of 5,120 mg/L and further solution is prepared in Mueller-Hinton broth. Outbred female CD-1 mice, 7 to 8 weeks old and weighing 20 to 25 g, are used in the experiments. Eight dose combinations are used. For the thigh-infected animals, the combinations of Ceftazidime and Avibactam are 16/4, 8/1, 64/32, and 2/128 mg/kg. For the lung-infected mice, combinations of 32/16, 4/2, 128/8, and 1/64 mg/kg of the respective constituents are used. These combinations are chosen in order to detect possible pharmacokinetic interactions between the two compounds (Ceftazidime and Avibactam) and to cover a wide range of doses of each compound. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Molecular Formula | C7H11N3O6S |
| Molecular Weight | 265.244 |
| Exact Mass | 265.036865 |
| PSA | 139.61000 |
| LogP | -3.20 |
| Index of Refraction | 1.679 |
| Storage condition | -20°C |