84605-18-5
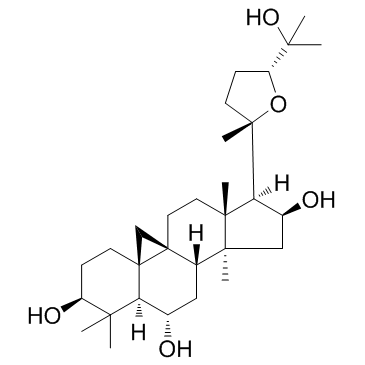
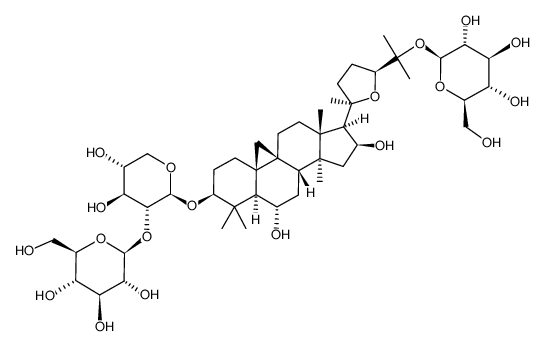
| Name | cycloastragenol |
|---|---|
| Synonyms |
9,19-Cyclolanostane-3,6,16,25-tetrol, 20,24-epoxy-, (3β,6α,9β,16β,20R,24S)-
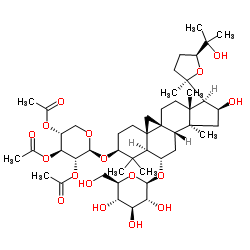
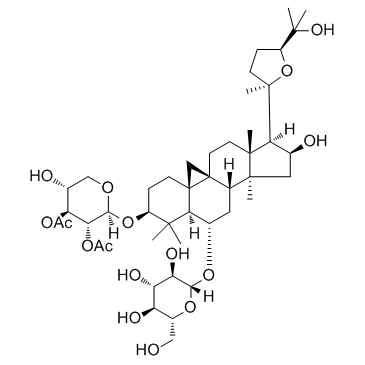
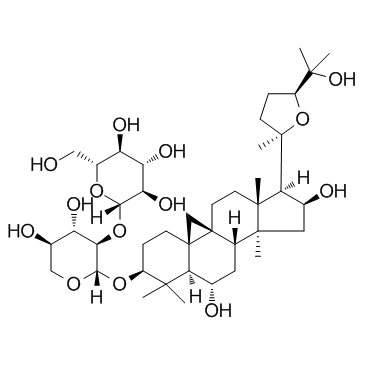
Astramembrangenin Cycloastragenol (3β,6α,9β,16β,20R,24S)-20,24-Epoxy-9,19-cyclolanostane-3,6,16,25-tetrol Cyclogalegigenin cycloartenol trans-ferulate Cycloartenol ferulic acid ester CYCLOGALEGENIN Oryzanol A 3-O-ferulylcycloartenol cycloartenyl ferulate Ring astragalus alcohol |
| Description | Cycloastragenol, a natural tetracyclic triterpenoid, was first identified when screening Astragalus membranaceus extracts for active ingredients with antiaging properties. IC50 value:Target:In vitro: In the study of Cycloastragenolon the treatment of degenerative diseases, the result showed that first-pass intestinal metabolism of cycloastragenol might occur upon passage through the intestinal epithelium. Cycloastragenol underwent extensive metabolism in rat and human liver microsomes with only 17.4% and 8.2%, respectively, of the starting amount of Cycloastragenol remaining after 30 min of incubation [1]. The present study demonstrates that cycloastragenol stimulates telomerase activity and cell proliferation in human neonatal keratinocytes. In particular, cycloastragenol promotes scratch wound closure of human neonatal keratinocyte monolayers in vitro [3]. In vivo: Rats were treated with Cycloastragenol (40 mg·kg- 1·d- 1) for 7 days to induce hepatic microsomal enzyme. The result showed that compared with the control, cycloastragenol obviously activated CYP2E1, and remarkably inhibited CYP3A4 [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 617.2±55.0 °C at 760 mmHg |
| Molecular Formula | C30H50O5 |
| Molecular Weight | 490.715 |
| Flash Point | 327.1±31.5 °C |
| Exact Mass | 490.365814 |
| PSA | 90.15000 |
| LogP | 3.82 |
| Vapour Pressure | 0.0±4.0 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Storage condition | -20°C |
| HS Code | 2942000000 |
|---|
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2942000000 |
|---|