577784-91-9
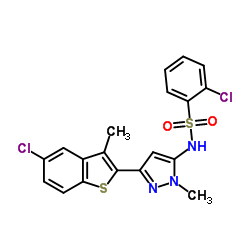
| Name | 2-chloro-N-[5-(5-chloro-3-methyl-1-benzothiophen-2-yl)-2-methylpyrazol-3-yl]benzenesulfonamide |
|---|---|
| Synonyms |
RNA Polymerase III Inhibitor
2-Chloro-N-[3-(5-chloro-3-methyl-1-benzothiophen-2-yl)-1-methyl-1H-pyrazol-5-yl]benzenesulfonamide N1-[3-(5-chloro-3-methylbenzo[b]thiophen-2-yl)-1-methyl-1H-pyrazol-5-yl]-2-chlorobenzene-1-sulfonamide Benzenesulfonamide, 2-chloro-N-[3-(5-chloro-3-methylbenzo[b]thien-2-yl)-1-methyl-1H-pyrazol-5-yl]- ML-60218 |
| Description | ML-60218 is a broad-spectrum RNA pol III inhibitor, with IC50s of 32 and 27 μM for Saccharomyces cerevisiae and human. ML-60218 disrupts already assembled viroplasms and to hamper the formation of new ones without the need for de novo transcription of cellular RNAs[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Combination of SAHA and ML-60218 produces enhanced suppression of proliferation in human pancreatic adenocarcinoma by impairing cell cycle progression and inducing apoptosis. ML-60218 reverses SAHA-stimulated tRNA expression in PANC-1 and BxPC-3 cells. ML-60218 enhances the ability of HDAC inhibitors to induce apoptosis and cell cycle arrest[2]. In in vitro transcription assays with purified double-layered particles (DLPs), ML-60218 shows dose-dependent inhibitory activity, indicating the viral nature of its target. ML-60218 is found to interfere with the formation of higher-order structures of VP6, the protein forming the DLP outer layer, without compromising its ability to trimerize. Electron microscopy of ML-60218-treated DLPs shows dose-dependent structural damage. ML-60218-mediated (10 μM ) viroplasm disruption causes NSP5 dephosphorylation[3]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 632.3±65.0 °C at 760 mmHg |
| Molecular Formula | C19H15Cl2N3O2S2 |
| Molecular Weight | 452.377 |
| Flash Point | 336.2±34.3 °C |
| Exact Mass | 450.998260 |
| PSA | 100.61000 |
| LogP | 6.83 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.715 |