36148-89-7
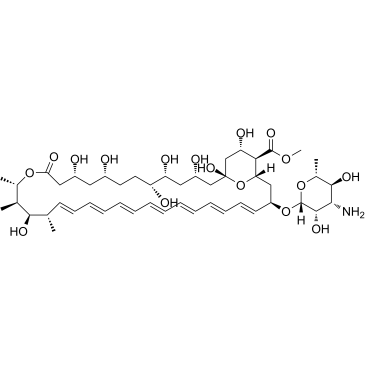
| Name | amphotericin B methyl ester |
|---|---|
| Synonyms |
14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid, 33-[(3-amino-3,6-dideoxy-β-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-, methyl ester, (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-
35-Deoxy Amphotericin B Methyl methylamphotericin b methyl (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,6-dideoxy-β-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylate Methyl (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,6-dideoxy-β-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14, 39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylate |
| Description | Amphotericin B methyl ester is the methyl ester derivative of the polyene antibiotic Amphotericin B (A634250). Amphotericin B methyl ester is the cholesterol-binding compound possesses significant antifungal activity. Amphotericin B methyl ester disrupts HIV-1 particle production and potently inhibits HIV-1 replication[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Amphotericin B methyl ester inhibits HIV-1 particle production with no significant effect on Gag binding to the plasma membrane, Gag association with lipid rafts, or Gag multimerization[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1102.6±65.0 °C at 760 mmHg |
| Molecular Formula | C48H75NO17 |
| Molecular Weight | 938.106 |
| Flash Point | 620.6±34.3 °C |
| Exact Mass | 937.503479 |
| PSA | 308.61000 |
| LogP | 1.63 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.602 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |