87134-87-0
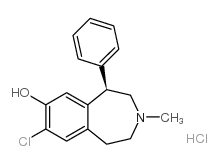
| Name | (Z)-but-2-enedioic acid,(5R)-8-chloro-3-methyl-5-phenyl-1,2,4,5-tetrahydro-3-benzazepin-7-ol |
|---|---|
| Synonyms |
MFCD00069249
unii-3t51j24n1f SCH23390 |
| Description | SCH-23390 maleate (R-(+)-SCH-23390 maleate) is a potent and selective dopamine D1-like receptor antagonist with Kis of 0.2 nM and 0.3 nM for the D1 and D5 receptor, respectively. SCH-23390 maleate is a potent and high efficacy human 5-HT2C receptor agonist with a Ki of 9.3 nM. SCH-23390 maleate also binds with high affinity to the 5-HT2 and 5-HT1C receptors. SCH-23390 maleate inhibits G protein-coupled inwardly rectifying potassium (GIRK) channels with an IC50 of 268 nM[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
D1 Receptor:0.2 nM (Ki) D5 Receptor:0.3 nM (Ki) 5-HT2C Receptor:9.3 nM (Ki) GIRK:268 nM (IC50) |
| In Vitro | SCH-23390 (1 μM) treatment reverses the inhibitory effects of Isosibiricin on NLRP3 expression and the cleavages of caspase-1 and IL-1β in the LPS-induced BV-2 cells. SCH-23390 could reverse the Isosibiricin-mediated inhibition of the NLRP3/caspase-1 inflammasome pathway[4]. |
| In Vivo | SCH-23390 can abolish generalized seizures evoked by the chemoconvulsants: pilocarpine and soman. SCH-23390 has also been used in studies of other neurological disorders in which the dopamine system has been implicated, such as psychosis and Parkinson's disease. Apart from the study of neurological disorders, SCH-23390 has been extensively used as a tool in the topographical determination of brain D1 receptors in rodents, nonhuman primates, and humans[1]. SCH-23390 is a very short-acting compound with an elimination half-life of around 25 min following administration of 0.3 mg/kg i.p. in the rat[1]. SCH-23390 augments dopamine-induced ductus constriction in CD-1 mouse vessels under newborn O2 conditions[5]. |
| References |
| Boiling Point | 414.7ºC at 760 mmHg |
|---|---|
| Molecular Formula | C21H22ClNO5 |
| Molecular Weight | 324.24500 |
| Flash Point | 204.6ºC |
| Exact Mass | 323.08400 |
| PSA | 23.47000 |
| LogP | 4.40530 |